N Dermatol Online. 2011; 2(2): 52-57
Conflicts of interest: None
SPONGIOTIC DERMATITIS WITH A MIXED INFLAMMATORY INFILTRATE OF LYMPHOCYTES, ANTIGEN PRESENTING CELLS, IMMUNOGLOBULINS AND COMPLEMENT
Abreu Velez Ana Maria1, Loebel Anne M.2, Howard Michael S.1
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA,
2Augusta Centre for Dermatology, Augusta, Georgia, USA
Corresponding author: Dr. Ana Maria Abreu Velez e-mail: abreuvelez@yahoo.com
How to cite an article: Abreu Velez AM, Loebel AM, Howard MS. Spongiotic dermatitis with a mixed inflammatory infiltrate of lymphocytes, antigen presenting cells, immunoglobulins and complement. Our Dermatol Online 2011; 2(2): 52-57.
Abstract
Background: The clinical and histological presentation of spongiotic dermatitis and its inflammatory infiltrates warrant further investigation. In this case documentation of a patient with cutaneous spongiotic reactivity, we aim to characterize antigen presenting cells, as well as the skin-specific cutaneous lymphocyte antigen population by multiple techniques. Case report: A 30 year old Caucasian female presented with a two week history of blistering and erosions around the vaginal, rectal and axillary areas. Material and Methods: We utilized hematoxylin and eosin histology, direct immunofluorescence, immunohistochemistry and confocal microscopy methods to evaluate the immune reaction patterns of the cutaneous inflammatory cells. Results: In the primary histologic areas of spongiotic dermatitis, a mixed population of B and T lymphocytes was seen. Ki-67 antigen proliferative index staining was accentuated in these areas, correlating with the presence of large numbers of epidermal and dermal antigen presenting cells. Among the antigen presenting cell population, we detected strong positivities with CD1a, Factor XIIIa, myeloid/hystoid antigen, S100, HAM-56, and CD68. Interestingly, immunoglobulins G, D and M and Complement factors C1q and C3 were also strongly expressed in antigen presenting cell areas, including positivity within the spongiotic epidermis and around dermal vessels. Conclusions: We document a heterogeneous population of B and T lymphocytes and the presence of multiple classes of antigen presenting cells, immunoglobulins and complement in and surrounding histologically spongiotic areas; these findings further correlated with increased levels of expression of Ki-67.
Key words: antigen presenting cells; B and T lymphocytes; endothelial cells; Ki-67; complement; immunoglobulins
Abbreviations and acronyms: Confocal microscopy (CFM), immunohistochemistry (IHC), direct immunofluorescence (DIF), hematoxylin and eosin (H&E), antigen presenting cells (APCs)
Introduction
Eczema is a common skin condition, histologically manifested as a spongiotic dermatitis. Patients experience intense pruritis that, if not controlled, can lead to secondary excoriation, with resultant infection and scarring. The first manifestation of this condition may occurs at young ages. Eczema predilects male patients at all ages [1-3]. Classically, it presents on the abdomen, chest or buttocks. Head and scalp presentations are unusual. A hereditary component has been postulated to contribute to the disease etiopathogenesis. An individual affected with eczema experiences itching or pain; these symptoms are accompanied by cutaneous inflammation, clinically manifested as the classic skin rash [1-3]. The rash may also develop fluid-filled blisters [1-3]. Other clinical causes of spongiotic dermatitis include allergic contact or systemic reactions to foods, plants, metals, dyes and medications. Infants may develop spongiotic dermatitis via an allergic contact diaper rash. Other clinical causes of a spongiotic dermatitis include environmental irritants, perfumes, smoke, and solvents; stress, hormone fluctuations, exposure to UVA/UVB solar radiation (especially if the patient is photosensitive), and climate changes [1-3]. Spongiotic dermatitis may initially be identified by its characteristic erythematous rash. If untreated, the condition may progresses and become chronic; the rash may darken in color and become rough and crusty. Exacerbated spongiotic dermatitis may display vesicles (small blisters filled with fluid) or bumpy, erythematous skin with pronounced pruritis. Topical medications are thus used to reduce both itching and inflammation. If the symptoms can be controlled, the disease progression will often be halted; thus, the possibility of permanent scarring is minimized [1-3]. If presenting pruritis is not accompanied by a significant rash, then a menthol-based cream or lotion may be utilized [1-3]. If the pruritis is thus not controlled, or if severe symptoms exist, then a topical corticosteroid may be prescribed. The topical corticosteroid will address both pruritis and inflammation. If topical treatments are ineffective, a prescription for an oral corticosteroid such as prednisone may be given to the patient. Some patients also report that taking Vitamin A or fish oil has provided relief from symptoms. Keeping the affected area moist with any kind of non-irritating lotion or cream is useful in reducing irritation. In our patient, a topical moisturizer and topical corticosteroid were prescribed with improvement of her lesions [1-3].
Case Report
A 30 year old Caucasian female presented in consultation to the dermatologist with two week history of blistering and erosions in the vaginal, rectal and axillary areas. The clinical history was relevant for childhood allergies. No previous adult history of allergies to food, deodorants, sanitary towels or cosmetics was noted. The clinical examination demonstrated vesicles and erosions in erythematous, edematous areas. The history and clinical examinations for sexually transmitted diseases were negative.
Methods
We performed two lesional skin biopsies from clinical blisters. The first biopsy was fixed in 10% buffered formalin, and submitted for hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) examination, as well as for immunohistochemistry (IHC). The second biopsy was placed in Michel’s transport medium, and submitted for direct immunofluorescence (DIF). The H&E, IHC and DIF studies and stains were performed as previously described [4-8].
Results
Examination of the H&E tissue sections demonstrated diffuse, florid epidermal spongiosis. Serum scale crust was present, and intraepidermal Langerhans cell microabscesses were noted. Early evidence of a subepidermal blistering disorder was seen, although frank blister formation was not observed. The dermis displayed a florid, superficial and deep, perivascular and interstitial infiltrate of lymphocytes, histiocytes, eosinophils, neutrophils and mast cells. Plasma cells were rare. No definitive evidence of an infectious, or a neoplastic process was observed. Focal, dermal perivascular leukocytoclastic debris was noted, but frank vasculitis was not appreciated (fig. 1,2,3). Direct immunofluorescence (DIF): was performed, and evaluated via the following grading system: (+) weak positive to (++++) strong positive, and (-) negative. Results of the DIF were IgG (+, Intracytoplasmic epidermal keratinocytic, and dermal perivascular); IgG4(-); IgA(-); IgM(-); IgD(-); IgE (-); Complement/C1q(++, Focal BMZ and superficial dermal perivascular); Complement/C3(++, Focal BMZ and superficial dermal perivascular); albumin (++++, diffuse, nonspecific dermal) and fibrinogen (++++, focal epidermal and florid, diffuse dermal) (fig 2,3). Confocal microscopy (CFM): We performed CFM examinations utilizing standard 20X and 40X objective lenses; each photoframe included an area of approximately 440 x 330 μm. Images were obtained using EZ-1 image analysis software (Nikon, Japan).
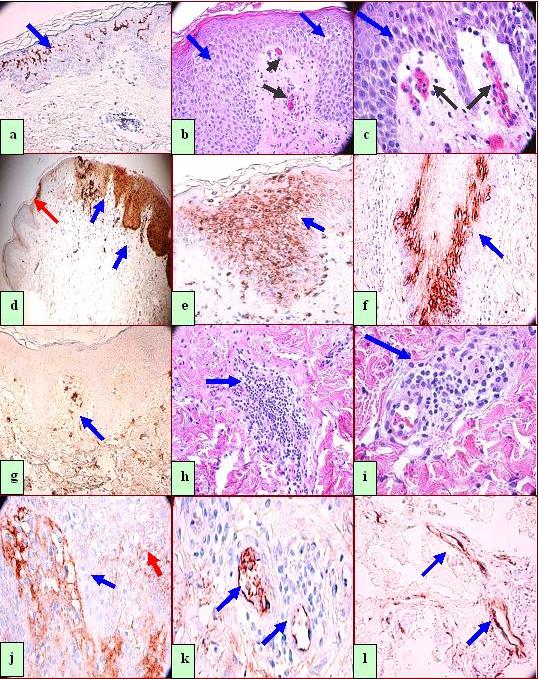 Figure 1.a CD1a positive cells in the epidermis by IHC (brown staining, blue arrow), b. H&E sections highlighting epidermal spongiosis, with widening of the spaces between keratinocytes (100X) (blue arrows). The black arrows highlight dermal papillary tip vascular microthrombi. c. Same as b, at higher magnification (400X). d. IHC showing strong positivity to myeloid/histoid antigen in the area of spongiotic epidermis (brown staining; blue arrows), in contradistinction to markedly less staining at the specimen periphery, unaffected by the spongiosis (100X) (red arrow). e. Similar to d, at higher magnification (400X) Blue arrow indicates positive epidermal myeloid/histoid staining; red arrow indicates punctuate staining in a papillary dermal tip. f. Positive Complement/C3 IHC staining around a hair follicle periphery below the spongiosis (brown staining, blue arrow). g. HAM-56 positive IHC straining around papillary dermal tip vessels where the microthrombi in b and c were seen (brown staining, blue arrow). h and i H & E staining shows inflammation around dermal blood vessels at lower and higher magnifications, respectively (blue arrows). j. Positive IHC staining for Complement/C3 in the papillary dermis (blue arrow) and at the basement membrane zone of the skin (blue arrow); also, note fine, punctate deposits within the epidermis (red arrow). k and l. IgD positive IHC staining in deep papillary dermal blood vessels (brown staining, blue arrows).
|
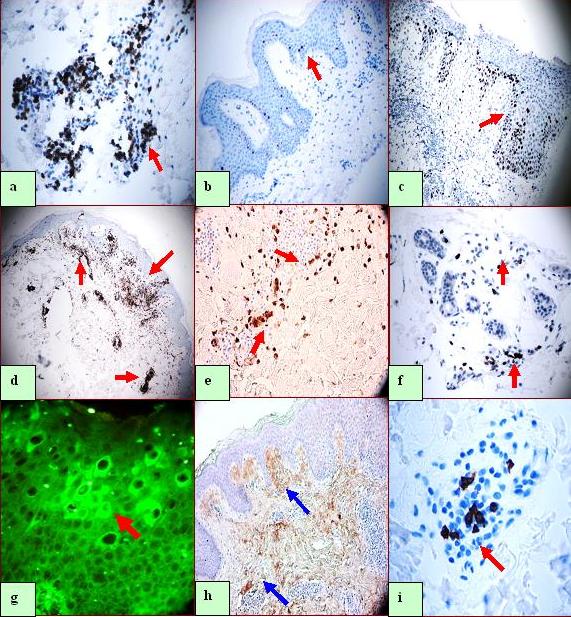 Figure 2.a, Epidermal CD3 IHC positive cells (dark brown staining, red arrow). b. and c. Positive Ki-67 IHC staining, in b on the non-spongitic edge of the skin biopsy (minimal brown staining, red arrow); in c, note the markedly increased staining in the histologically spongiotic area (dark brown staining, red arrow). d. CD45 positive IHC staining, accentuated in superficial and deep dermal areas cells subjacent to the spongiotic process (brown staining, red arrows) (40X). The CD45 positive staining included both CD3 and CD20 positive lymphocytes. e. Positive CD68 IHC staining on individual cells, grouped in the papillary dermis subjacent to the spongiosis (brown staining, red arrows). f. CD3 positive IHC staining on lymphocytes infiltrating eccrine glands subjacent to the spongiosis (brown staining, red arrows). g. DIF demonstrating positive staining with anti-human FITCI conjugated IgG, in perinuclear and cytoplasmic patterns in spongiotic epidermal keratinocytes (green staining, red arrow). h. Positive Complement/C1Q IHC staining, diffuse in the papillary dermis in surrounding papillary dermal tip blood vessels adjacent to the spongiotic process (brown staining, blue arrows). i. Positive CD8 IHC staining around the dermal papillary tip blood vessels (dark brown staining, red arrow).
|
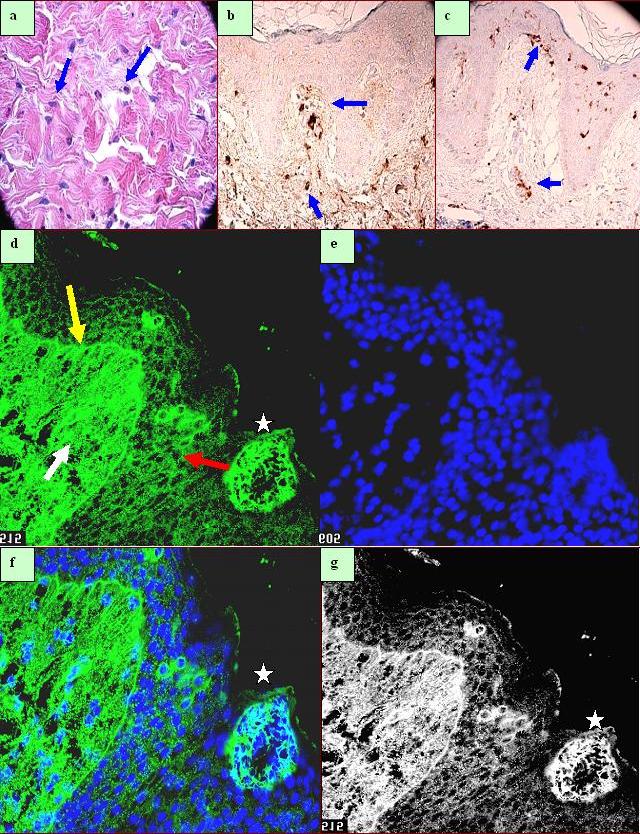 Figure 3.a. H & E, highlighting fibrohistiocytic cells in the dermis (grey cells, blue arrows). b Many of the fibrohistiocytic cells were positive for Factor XIIIa (blue arrows). c. Positive S100 IHC staining on epidermal and dermal Langerhans cells (blue arrows). d through g Confocal microscopy(CFM). In d and f, staining for FITCI conjugated anti-human fibrinogen, showing in d shaggy linear staining at the basement membrane zone of the dermal/epidermal junction (yellow arrow). The white stars highlight positive net-like staining, likely representing fibrinogen deposition within epidermal Langerhans microabscesses. The red arrow highlights scattered fibrinogen positive cells in the epidermis. The white arrow shows a strong papillary dermal positivity, subjacent to the primary epidermal spongiotic area. e. Shows positive epidermal nuclear counterstaining with Dapi (dark blue). f, Combined CFM staining of fibrinogen (green staining) and Dapi(blue staining). g, similar to f in black and white relief, highlighting positive fibrinogen staining on epidermal cells and cell junctions
|
Discussion
Spongiotic dermatitis (SD) is often encountered in routine dermatology and dermatopathology practice. Spongiosis is a term used to describe the dermatopathologic appearance of an epidermis impacted by intercellular edema. Resultant spaces are present between epidermal keratinocytes, which may progress to intraepidermal vesiculation. The pathophysiologic mechanism of spongiosis remains unknown. It has been proposed that keratinocyte apoptosis induced by T-cells affects transmembrane proteins involved in cell to cell adhesion; the protein alterations may then be responsible for development of spongiosis via dermal hydrostatic pressure [1-3]. Additional histologic features of spongiotic dermatitides include serum crust, lymphocytic exocytosis, and Langerhans cell microabscesses within the epidermis. Prior studies have attempted to characterize the inflammatory infiltrates present in spongiotic dermatitis [9-10]. A predominant T lymphocyte population has been reported [9-10]. In this study, our findings were in agreement with several authors because we also detected a large population of T lymphocytes. However, we also found several CD1a, factor XIIIa, HAM-56 and CD68 positive cells. Consistent with our findings, other authors have also reported that immunoglobulin D may play a pathophysiologic role [11]. Interestingly, we found robust B and T lymphocyte activity, and a prominent antigen presenting cell (APC) population. Further, the reactive cell and cytokine combination has not been previously highlighted in spongiotic dermatitides. The APCs participate in the initiation of the inflammatory process in various immune-mediated dermatoses, via activation of antigen specific T lymphocytes. The skin contains several different subsets of APCs. Non-professional APCs do not constitutively express the MHC class II proteins required for interaction with naive T cells; these are expressed only upon stimulation of the non-professional APC by certain cytokines such as gamma interferon (IFN-γ). Non-professional APCs in the skin include dermal fibrohistiocytic cells and vascular endothelial cells. In our patient, we detected multiple professional and non-professional APCs, as well as broad B and T activated lymphocytic populations in relevant areas. Indeed, the myeloid/hystiocyte antigen (reactive with human cytoplasmic L1 antigen, or calprotectin) was very reactive in the zone of epidermal spongiosis, as well as in the dermis in proximity to this process [13,14]. The fact that we found complement as well as immunoglobulins in the inflamed area indicates that the immune response in an spongiotic dermatitis may be more complex than currently thought. Thus, we recommend future studies to further investigate these findings. In our case the primary clinical cause of the spongiotic dermatitis was not determined with certainty. The patient was treated with topical steroids and oral antihistamines, with subsequent complete improvement of her dermatosis.
Acknowledgement
Jonathan S. Jones, HT(ASCP) and Lynn K. Nabers HT, HTL(ASCP) at GDA for excellent technical assistance.
REFERENCES
1. Gupta K: Deciphering spongiotic dermatitides. Indian J Dermatol Venereol Leprol. 2008,74: 523-526. 2. Jaspars EH: The immune system of the skin and stereotyped reaction patterns in inflammatory skin diseases. Ned Tijdschr Geneeskd. 2006; 150: 948-955. 3. Houck G, Saeed S, Stevens GL, Morgan MB: Eczema and the spongiotic dermatoses: a histologic and pathogenic update. Semin Cutan Med Surg. 2004; 23: 39-45. 4. Abreu Velez AM, Smith JG, Howard MS: IgG/IgE bullous pemphigoid with CD45 lymphocytic reactivity to dermal blood vessels, nerves and eccrine sweat glands. North Am J Med Sci 2010; 2: 538-541. 5. Abreu Velez AM, Klein AD, Howard MS: Junctional adhesion molecule overexpression in Kaposi varicelliform eruption skin lesions as a possible herpes virus entry site. North Am J Med Sci 2010; 2: 433-437. 6. Abreu Velez AM, Howard MS, Smoller BR: Atopic dermatitis and possible polysensitization, monkey esophagus reactivity. North Am J Med Sci. 2010; 2: 336- 340. 7. Abreu Velez AM, Howard MS, Brown VM: Antibodies to piloerector (arrector pili) muscle in a case of the lupus/lichen planus overlap syndrome. North Am J Med Sci. 2010; 2: 276-280. 8. Howard MS, Yepes MM, Maldonado JG, Villa E, Jaramillo A, et al: Abreu Velez AM. Broad histopathologic patterns of non-glabrous skin and glabrous skin from patients with a new variant of endemic pemphigus foliaceus (part 1). J Cutan Pathol. 2010. 37: 222-230. 9. Deguchi M, Aiba S, Ohtani H, Nagura H, Tagami H: Comparison of the distribution and numbers of antigenpresenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease. Arch Dermatol Res. 2002; 294: 297-302. 10. Deguchi M, Ohtani H, Sato E, Naito Y, Nagura H, et al:. Proliferative activity of CD8(+) T cells as an important clue to analyze T cell-mediated inflammatory dermatoses. Arch Dermatol Res. 2001; 293: 442-447. 11. Jacobsen JT, Lunde E, Sundvold-Gjerstad V, Munthe LA, Bogen B: The cellular mechanism by which complementary Id+ and anti-Id antibodies communicate: T cells integrated into idiotypic regulation. Immunol Cell Biol. 2010; 88: 515-22. 12. Cobbold SP, Adams E, Nolan KF, Regateiro FS, Waldmann H: Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol Rev. 2010; 236: 203-218. 13. Brandtzaeg P, Dale I, Gabrielsen TO: The leukocyte protein L1 (calprotectin): usefulness as an immunohistochemical marker antigen and putative biological function. Histopathology. 1992; 21: 191-196. 14. Rugtveit J, Scott H, Halstensen TS, Norstein J, Brandtzaeg P; Expression of the L1 antigen (calprotectin) by tissue macrophages reflects recent recruitment from peripheral blood rather than upregulation of local synthesis: implications for rejection diagnosis in formalin-fixed kidney specimens. J Pathol. 1996; 180: 194-199.
Comments are closed.