Kerion Celsi: A report of two cases due to Microsporum gypseum and Trichophyton tonsurans
Edoardo Torres-Guerrero1, Erick Martínez-Herrera1, Stefanie Arroyo-Camarena2, Carlos Porras3, Roberto Arenas1
1Mycology section, “Dr. Manuel Gea González” Hospital, Mexico City, Mexico, 2Dermatology Department “Dr. Manuel Gea González” Hospital, Mexico City, Mexico, 3Mycology section, Institute of Dermatology and Skin Surgery “Prof. Dr. Fernando A. Cordero C.” Guatemala City, Guatemala
ABSTRACT
Tinea capitis is a scalp fungal infection involving the hair. Inflammatory cases are usually caused by zoophilic and geophilic species of the genus Microsporum and Trichophyton, and are almost always seen in children. The most effective treatments are with Griseofulvin, itraconazole and terbinafine. We report two cases in children 5 and 7 years old, in which Microsporum gypseum and Trichophyton tonsurans were isolated.
Key words: Kerion Celsi; Dermatophytes; Microsporum; Trichopyton
INTRODUCTION
Tinea capitis is an infection caused by dermatophyte fungi that affects the scalp and hair; the main pathogens are species of the genera Microsporum and Trichophyton [1–3]. According to Sarabi and Khachemoune, it can be classified as anthropophilic (T. rubrum, T. tonsurans, M. audouinii, T. violaceum), zoophilic (M. (M. gypseum, M. fulvum) [2]. It is almost exclusively in children and rarely occurs after puberty [4].
Inflammatory tinea capitis, also known as kerion celsi, is a less common variety, about 13-15% of dermatophytosis of the scalp, usually caused by zoophilic dermatophytes like M. canis, T. mentagrophytes var. mentagrophytes [2,5], T. verrucosum, T. megninii (T. rosaceum), T. violaceum and T. soudanense; however, T. tonsurans has been documented which may be related to the geographic distribution of microorganisms [4,6]. It is clinically characterized by a solitary and usually very painful well-circumscribed inflammatory lesion, with broken-off hair, and purulent discharge from multiple openings [7].
The inflammation is caused by immunological mechanisms; the lesions may be secondarily infected with bacteria (S. aureus) [2] and also, lymphadenopathy [3] may be present.
CASE REPORT
Case 1
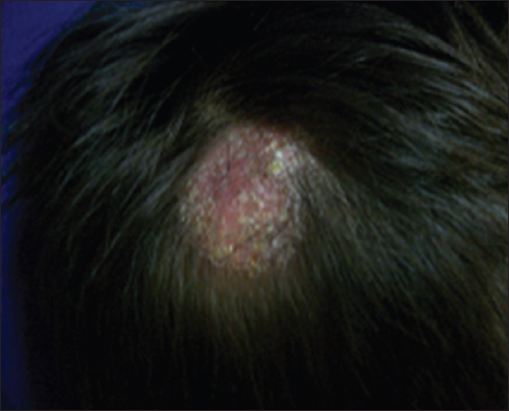
A 5 year old boy, presented on the parietal region a 4X4 cm indurated, erythematous scaling plaque with alopecia, and sharp edges; he previously used sulfur soap without improvement. In addition, the patient reported itching and denied fever (Fig. 1).
A superficial scraping of the lesion was performed with a #15 scalpel blade for direct examination with potassium hydroxide and dimethylsulfoxide (KOH-DMSO) where hyaline hyphae and ectothrix hair invasion were visualized.
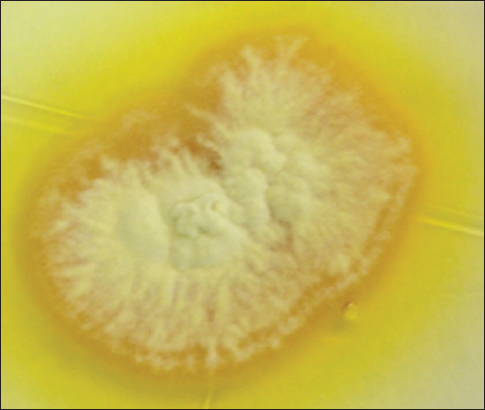
Mycosel® agar culture (Sabouraud with cycloheximide and chloramphenicol) showed a powdery beige colony, suggestive of Microsporum gypseum and was confirmed by microscopic examination (Fig. 2).
The patient was treated with griseofulvin (10 mg/kg), with complete resolution, after 45 days. Gastrointestinal symptoms associated to the drug, were the reason to reduce the dose, and increasing the duration of treatment.
Case 2
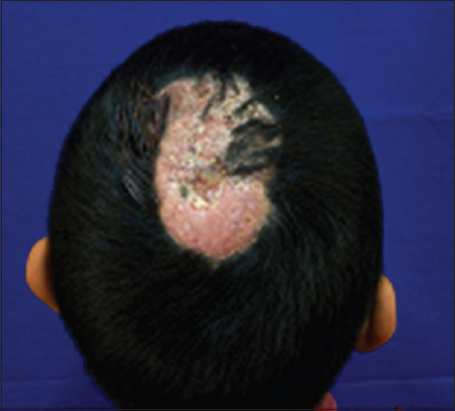
A 7 year old boy with a lesion on the occipital region of his scalp, characterized by an erythematous plaque of 2.5 cm in diameter, indurated, with alopecia, yellowish scales, with well-defined edges, itchy, and without fever (Fig. 3).
Mycosel® agar showed a yellowish color and radiated colony with powdery appearance, suggestive of Trichophyton tonsurans var. sulfureum, confirmed by microscopic examination. The lesion resolved spontaneously within 15 days (Fig. 4).
DISCUSSION
Inflammatory tinea capitis or kerion celsi usually occurs in children, starting as a dried form; the inflammatory response is due to immunological mechanisms of the patient [3,8].
The most frequent strains that infect hair and produce inflammatory conditions are zoophilic (M. canis, T. mentagrophytes var. mentagrophytes and T. verrucosum), geophilic (M. gypseum), and sometimes, anthropophilic dermatophytes, as T. tonsurans [4].
The typical clinical presentation is an inflammatory mass draining pus from multiple openings, usually painful with pressure; and there is satellite lymphadenopathy with or without fever [1].
Epidemiology may vary depending on the geographical area; the most common etiologic agents in Mexico are M. canis (80%), T. tonsurans (15%) and sporadically M. gypseum, T. mentagrophytes var. mentagrophytes, T. verrucosum and T. violaceum [3]. It is important to note that in the decade of 1960 to 1970 the most prevalent tinea capitis in Mexico was trichophytic type (Trichophyton tonsurans) and later changed to a predominance of M. canis [9]. In the United States the most common etiologic agent is T. tonsurans, which overtook M. canis in the last 100 years [10].
Other pathogens can be isolated in different regions of the world, as in the Caribbean and Europe (M. audouinii, T. tonsurans, T. violaceum and M. canis), in China and Japan (M. ferrugineum), Rusia (T. violaceum) [3], moreover there may even be variations within the same area, as in a study by Arenas and Torres in which predominance of T. tonsurans was observed in rural areas of Dominican Republic, while M. audouinii was more common in urban areas of the same country [11]; as in the case of Europe, where in England, T. tonsurans is the most common agent for tinea capitis, M. canis in central and southern Europe and T. violaceum in Greece and Belgium [12].
In the first case of this study we isolated Microsporum gypseum, known in the literature as a causative agent of inflammatory tinea capitis.
M. gypseum is an unusual geophilic pathogen, with a frequency of 0.8% of cases of tinea capitis [13,14]. It occurs in children up to 75% of cases, primarily causing tinea corporis, but also tinea capitis, tinea barbae, tinea faciei and onychomycosis. In the scalp it presents as a scaling plaque of alopecia (pseudo-alopecia); pustules, abscesses and golden-yellow crusts are seen in the inflammatory variety [14].
Macroscopic aspect of the culture shows powdery colonies of rapid growth and a light cinnamon color; the reverse may be yellowish. Microscopy shows fusiform and thin-walled macroconidia with less than 6 cells, arranged in groups or clusters. Microconidia are sessile, smooth-walled and club shaped.
In some parts of the world, the most frequently documented agent is T. mentagrophytes; in a study by Palacios et al. in Spain, a predominance of T. mentagrophytes var. granulosum was observed and they found only one case caused by M. gypseum in a child older than 16 years [15]; whereas in a study in Yucatan, Mexico, among 114 cases of tinea capitis, both clinical forms (dry and inflammatory) were found in equal proportion, M. canis and T. tonsurans as the main etiological agents and T. mentagrophytes, M. canis, T. tonsurans and M. gypseum for the inflammatory variety [4]. While in a case series study of 19 children with inflammatory tinea capitis with biopsy and mycological study, M. gypseum was found in 5% of cases [16].
Martinez and Arenas, in a previous study conducted in Guatemala, found the inflammatory form in 36.7% of 60 patients with tinea capitis. In the samples collected for this study, the most frequently isolated etiologic agents were M. canis (69.8%), followed by T. rubrum and M. gypseum (13.2% each) [17].
In the second case Trichophyton tonsurans was isolated, which morphologically corresponded to the variety sulfureum.
There have been reports of T. tonsurans in Mexico; in a study of nine cases of inflammatory fungal infections (trichophytic granuloma and kerion), it was found that three cases were caused by T. tonsurans and one case by T. rubrum [4].
In an outbreak in 5 members of the same family studied in a dermatological center of Mexico City, the inflammatory form was documented in all cases and the causative agent was T. tonsurans [18].
T. tonsurans is a common agent of tinea capitis and tinea corporis. It is the main pathogen of tinea capitis in the United States, Canada and England [19,20]. In Mexico it was only identified in 10% of all cases of tinea capitis and mainly acquired after contact with an infected person or with fomites [18].
This is an anthropophilic dermatophyte, so animals are never affected, occurring infection by contact from person to person [19].
The macroscopic study shows white-gray colonies, radiated and sometimes with a slight red – brown or yellow pigment on the back. On microscopic examination shows cigar-shaped macroconidia and numerous pyriform microconidia on the sides of the hyphae and in its terminal portion are arranged in a “Cross of Lorraine”.
Because of this variation in the frequency of etiologic agents, the importance of regular epidemiological studies is emphasized to present the current status of this clinical entity [9,16].
The first case caused by M. gypseum was treated with griseofulvin, which showed good therapeutic response. In the second case, due to T. tonsurans, there was no need to prescribe antifungal therapy because of the spontaneous resolution.
Inflammatory dermatophytosis may cause permanent alopecia, therefore should always be treated by a doctor. As important historical antecedent, when no treatments for dermatophytosis were available, cases of scarring alopecia secondary to kerion celsi were relatively frequently.
Currently, the most appropriate treatment is griseofulvin, 10 to 20 mg/kg per day and in resistant cases, up to 30 mg for 8 to 12 weeks. Other successful alternatives are itraconazole and terbinafine; although fluconazole has been used, it is not very effective. Terbinafine can be used to treat infections of T. tonsurans and itraconazole for M. canis [21]. Prednisone is recommended along with antimycotics to treat the inflammation, and reduce the degree of scarring alopecia [1,16].
CONSENT
The examination of patients is conducted according to the Declaration of Helsinki principles. Written informed consent was obtained from the patient for publication of this case report.
REFERENCES
1. Arenas R, Micología Médica Ilustrada2011; 4ª ed. México: Mc Graw Hill; 61-91.
2. Sarabi K, Khachemoune A, Tinea capitis: A reviewDermatol Nursing 2007; 19: 525-9.
3. Bonifaz A, Micología Médica Básica2010; 3ª ed. México: Mc Graw Hill; 101-119.
4. López–Bárcenas A, Atoche–Diéguez C, Cerón J, Rebollo–Domínguez N, Arenas R, Epidemiologia de la tiña de la cabeza en Yucatán. Estudio de 114 casosDCMQ 2009; 7: 87-90.
5. Asbati M, Bell Smythe A, Cavallera E, Querion de Celso ulcerado por Trichopyton mentagrophytes, var mentagrophytesRev Soc Ven Microbiol 2002; 22: 144-6.
6. Isa – Isa R, Arenas R, Isa M, Inflammatory tinea capitis: Kerion, dermatophytic granuloma, and mycetomaClin Dermatol 2010; 28: 133-136.
7. Bussy RF, Gatti CF, Guardia CP, Fundamentos en Dermatología ClínicaBuenos Aires J 2011; 30-1.
8. Medina D, Del Carmen M, Fernández R, Arenas R, Bonifaz A, Tinea de la cabeza en adultos: Estudio clínico, micológico y epidemiológico de 30 casos en Ciudad de MéxicoPiel 2003; 18: 403-8.
9. Arenas R, Dermatofitosis en MéxicoRev Iberoam Micol 2002; 19: 63-7.
10. Gupta A, Summerbell R, Tinea Capitis” Med Micol2000; 28: 255-87.
11. Arenas R, Torres E, Amaya M, Rivera ER, Espinal A, Polanco M, Tinea capitis. Emergencia de Microsporum audouinii y Trichophyton tonsurans en la República DominicanaActas Dermosifilogr 2010; 101: 330-5.
12. Claire F, Changing face of tinea capitis in EuropeCurr Opin Infect Dis 2009; 22: 115-8.
13. Zhang R, Ran Y, Dai Y, Zhang H, Lu Y, A case of Kerion celsi caused by Microsporum gypseum in a boy following dermatoplasty for a scalp wound from a road accidentMed Mycol 2011; 49: 90-3.
14. Chanussot C, Arenas R, Querón de Celso por Microsporum gypseum en un niño de 5 añosDermatol Venez 2010; 48: 47-9.
15. Del Palacio A, Cuétara María, Valle A, González A, Almondarain I, Ramos M, Cambios epidemiológicos observados en un decenio en las dermatofitosis del hospital universitario “12 de Octubre” de Madrid: nuevas especies emergentes”Rev Iberoam Micol 1999; 16: 101-6.
16. Arenas R, Toussaint S, Isa Isa R, Kerion and dermatophytic granuloma. Mycological and histopathological findings in 19 children with inflamatory tinea capitis of the scalpInt J Dermatol 2006; 45: 215-9.
17. Martínez E, De León S, Pérez E, Pacheco A, Rivas E, Borjas C, Tinea capitis. Informe de 60 casos con parasitación pilar y/o agente causal confirmadoDCMQ 2009; 7: 98-101.
18. Rodríguez M, Padilla MC, Martínez JA, Tiña inflamatoria de la cabeza por Trichophyton tonsurans. Comunicación de 5 casos dentro de un mismo núcleo familiarRev Cent Dermatol Pascua 2006; 15: 26-30.
19. Carrillo Silvestri F, Campos González M, Barba Gómez F, Mayorga Rodríguez J, Epidemia familiar por Trichophyton tonsuransDermatología Rev Mex 1998; 42: 13-5.
20. Mayorga J, Espinoza Gómez R, Villarreal Parra I, García Vargas A, Tiña de la cabeza. Observaciones clínico – micológicas en 30 pacientesDermatol Rev Mex 1999; 43: 264-7.
21. Elewsky B, Tinea capitis: A current perspectiveJ Am Acad Dematol 2000; 1: 1-20.
Notes
Source of Support: Nil
Conflict of Interest: None declared.

Comments are closed.