DOI: 10.7241/ourd.20124.77 article in PDF
Our Dermatol Online. 2012; 3(4): 347-349
Date of submission: 09.07.2012 / acceptance: 09.08.2012
Conflicts of interest: None
HOW TO DIFFERENTIATE MUCINOUS ECCRINE CARCINOMA FROM CUTANEOUS METASTASIS OF BREAST CARCINOMA?
Mona Mlika1, Emna Boudabbous1, Aida Ayadi-Kaddour1, Lamia Kassar2, Faouzi El Mezni1
1Department of Pathology, Abderrahman Mami Hospital, University of Medicine. Tunis El Manar, Tunisia
2Department of Dermatology, Mahmoud El Matri Hospital, University of Medicine. Tunis El Manar, Tunisia
Corresponding author: Dr. Mona Mlika e-mail: mlika.zorgati.mona@hotmail.com
How to cite an article: Mlika M, Boudabbous E, Ayadi-Kaddour A, Kassar L, El Mezni F. How to differentiate mucinous eccrine carcinoma from cutaneous metastasis of breast carcinoma? Our Dermatol Online. 2012; 3(4): 346-348.
Abstract
Eccrine skin tumors are rare and represent only 0,05% of all cutaneaous neoplasms. They represent a pitfall especially with cutaneous metastases of carcinoma which are more frequent. We report the case of a 60-year-old woman presented with a frontal scalp mass whose histologic and immunohistochemical features concluded initially to a cutaneous metastasis of breast carcinoma. The diagnosis was reviewed because of the absence of a breast lesion. The final diagnosis was primary sweet gland carcinoma. Histologic distinction between cutaneous metastatic breast carcinoma and primary cutaneous adnexal neoplasms can be very challenging or even impossible. This case illustrates this difficulty and puts emphasis on the necessity of keeping in mind the distinctive features between these two entities.
Key words: metastatic breast carcinoma; eccrine mucinous carcinoma; pitfall
Introduction
Approximately 25% of the patients with breast cancer develop cutaneous metastases. The major differential diagnosis of cutaneous metastatic breast cancer is represented by sweat gland carcinoma which accounts for about 0, 05% of all cutaneous neoplasms [1,2]. Treatment and prognoses of these two entities differ radically making accurate histologic diagnosis mandatory. Indeed, the presentation of these two entities is often distinct. Cutaneous metastasis of breast carcinoma presents as multiple lesions in patients with a previous diagnosis of primary breast carcinoma, whereas, sweat gland carcinoma presents as a single cutaneous lesion in patients with unknown history of breast cancer. However, cutaneous metastases of breast carcinoma can be difficult to distinguish from sweat gland carcinoma when the diagnosis is based mainly on histologic features and the clinical circumstances are unknown by the pathologist.
We describe a new case of sweat gland carcinoma which presented a real diagnostic dilemma.
Case Report
A 60-year-old woman presented with a frontal scalp mass which appeared 6 months ago. The patient was asymptomatic and had no history of trauma. Physical examination revealed a painless mass measuring 0.5 cm. Incision biopsy was performed and microscopic findings consisted in a dermal malignant tumor proliferation arranged in clumps and lobules surrounded by an abundant mucoid stroma and separated by thin fibrous septa. Tumor cells were monomorphic, rounded with cytoplasmic vacuoles of mucus secretion and atypical nucleolated nuclei (Fig. 1a, 1b). Immunohistochemical findings showed a nuclear expression of estrogenic and progesteronic antigens (Fig. 1c). Tumor cells didn’t express HER2-Neu antigen (Fig. 1d). These microscopic findings were suggestive of a cutaneous metastasis of an eventual mucinous breast carcinoma. A mammography and a chest MRI were performed targeting the primary breast lesion and showed no breast lesion.
Facing these radiologic findings, we concluded to a cutaneous mucinous eccrine carcinoma. So that, the lesion was totally resected and the patient presented no complications after one year of follow up.
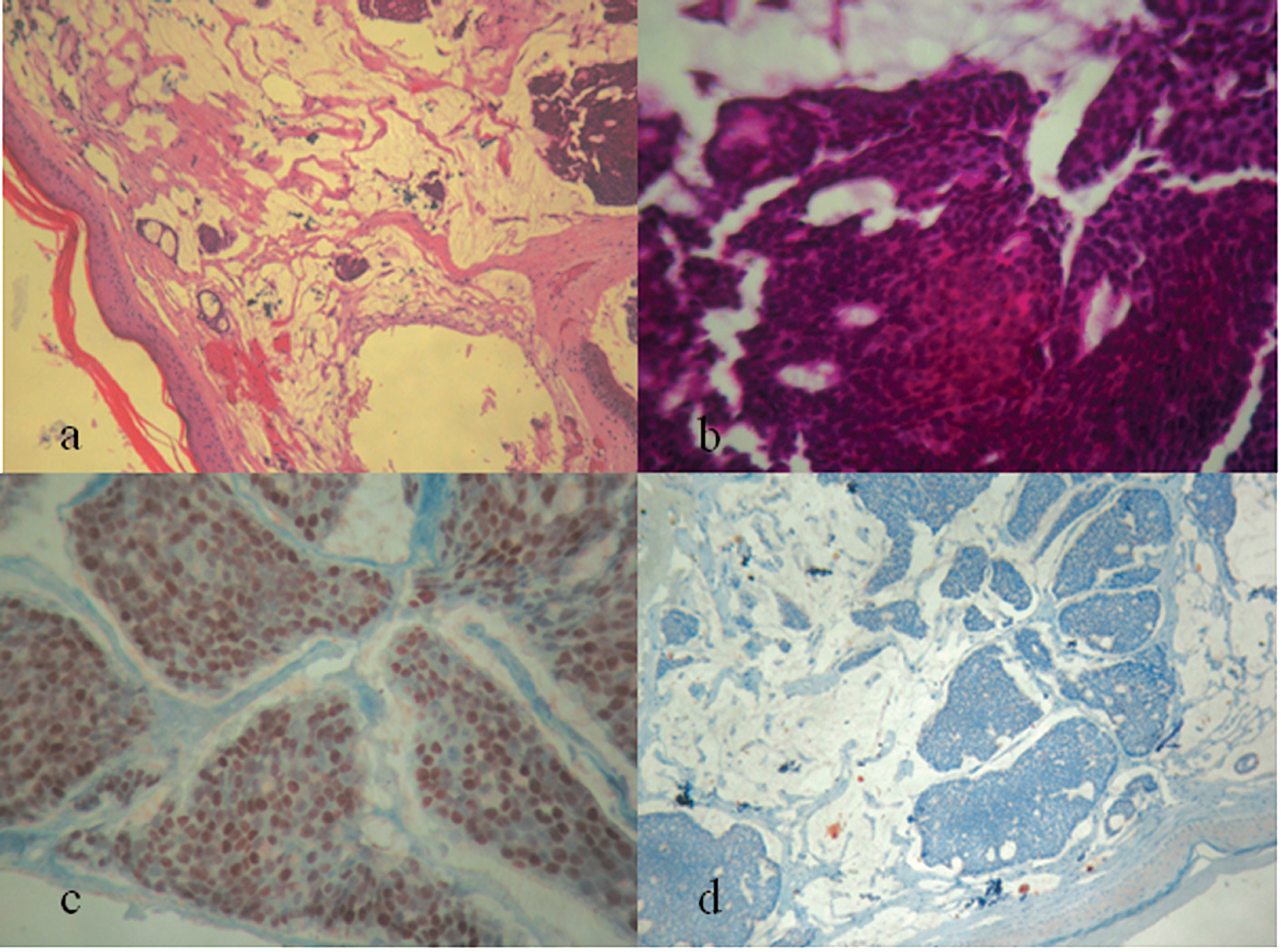 Figure 1. a) Dermal malignant tumor arranged in lobules (HE x 250), b) Tumor cells are monomorphic, rounded with cytoplasmic vacuoles of mucus secretion and atypical nucleolated nuclei. They are surrounded by an abundant mucoid stroma (HE x 400), c) Nuclear expression of estrogenic antigen (HE x 400), d) Absence of expression of Her2-neu by tumor cells (HE x 400).
|
Discussion
The distinction between adnexal tumors and cutaneous metastases of breast cancer may be difficult. Based on clinical characteristics, adnexal tumors are mainly observed as unique lesion in opposition to cutaneous metastasis of breast carcinoma which consists generally in multiple lesions that are observed in women with a past medical history of breast carcinoma. Otherwise, in some cases, when the breast cancer is indolent or when the pathologist hasn’t sufficient clinical informations, the microscopic differentiation between these two entities may be very challenging. The histologic similarities between these lesions have been attributed to their common embryologic derivation in particular their origin as ectodermal downgrowths from the epidermis. Microscopically, eccrine mucinous carcinoma is characterized by large pools of basophilic mucin which are compartmentalized by delicate fibrous septa, thereby creating a honeycomb pattern. Within the lakes of mucin are small “floating” islands and bizarre clusters of neoplastic epithelial cells, sometimes exhibiting a cribriform arrangement. The epithelial component is denser at the periphery of the tumor. Small glandular or tubular structures containing mucin or showing signs of apocrine secretion occur rarely. The small neoplastic cells are cuboidal, round, or oval with abundant cytoplasm that may be vacuolated. Nuclei are small with mild atypia. Mitoses are rare. The mucin is PAS positive, hyaluronidase and sialinase labile, and consists in nonsulphated acid mucopolysaccharides with sialic acid [3]. Otherwise, cutaneous metastatic mucinous carcinoma of the breast is characterized by proliferation of clusters of generally uniform, round cells with minimal amounts of eosinophilic cytoplasm, floating in lakes of mucus. Delicate fibrous septa divide the mucous lake into compartments. The cell clusters are variable in size and shape; sometimes with a tubular arrangement; rarely, they assume a papillary configuration. Atypia, mitotic figures and microcalcifications are not common [3]. Facing these histologic similarities, some authors searched for discriminating antibodies, so that, previous studies using antibodies to evaluate the expression of various proteins, including estrogen and progesteron receptors, anti-gross cystic disease fluid protein (BRST-2), carcino-embryonic antigen, S-100 protein and epidermal growth factor have shown trends in staining patterns that may be helpful. There has been no report to date of a single marker that reliably makes the distinction between these neoplasms. The diagnostic value of c-erb-B2 antibody have been explored showing an overexpression of this antigen in 33% of the eccrine mucinous carcinoma and in 20% of the breast carcinoma. This result made this antibody unreliable in differentiating both entities. A large study carried by Busam and coworkers evaluated the staining pattern of primary cutaneous sweat gland carcinoma and primary or metastatic breast carcinoma [4]. They found promising results with the epidermal growth factor receptor (EGF-R) which is a protein that has significant homology with HER-2. They showed that 81% of the sweat gland carcinomas were EGF-R positive, with a predominantly strong, diffuse membraneous pattern. On the other hand, only 17% of the metastatic breast carcinomas were EGF-R positive with a focal expression. Hiatt KM and colleagues tried to determine if EGF-R antibody could be applied for the histologic differentiation of metastatic breast carcinoma from primary cutaneous adnexal neoplasms. They compared cutaneous metastasis of breast carcinoma with the primary lesions which were known as over expressing HER-2 antigen. They found that 77 to 100% of HER-2 positive primary tumors maintained HER-2 expression in secondary localizations. While, among the 10% to 34% of breast carcinomas which over-expressed the HER-2 protein, only 3% cutaneous apocrine and eccrine neoplasms in this study had any HER-2 expression [5]. According to these results, in our case the tumor cells didn’t express HER2- Neu antigen. These results put emphasis on the fact that despite their similar morphology and embryologic derivations, the expression of EGF-R antigen in association with the differential staining pattern based on HER-2 expression suggests that cutaneous adnexal tumors and mammary glands carcinomas are nosologically different from each other. In another study conducted by Rollins-Raval and colleagues, a large panel of antibody was used to differentiate these neoplasms. This panel consisted in mammoglobin, p63 and three basal cytokeratins (CK5 , CK17 , CK14). The authors recommended the use of this panel to differentiate most cases of sweat gland carcinoma and ductal cutaneous metastases of breast carcinoma which were generally positive for mammoglobin and negative for p63, CK5, CK17 and CK14 [2].
Conclusion
The distinction between cutaneous metastatic breast carcinoma and primary cutaneous adnexal neoplasms is very difficult or even impossible. Microscopic appearance is quite similar and the distinctive antibodies are non consensual. Pathologists should keep in mind that these two entities may express hormone receptors and that the distinction might be enabled using some antibodies such as Her2-Neu, EGF-R or P63 antibodies.
REFERENCES
1. Hiatt KM, Pillow JL, Smaller BR: Her-2 expression in cutaneous eccrine and appocrine neoplsms. Mod Pathol. 2004;17:28-32.
2. Rollins-Raval M, Chivukula M, Tseng GC, Jukic D, Dabbs DJ: An immunohistochemical panel to differentiate metastatic breast carcinoma to skin from primary sweat gland carcinomas with a review of the literature. Arch Pathol Lab. 2011;135:975-83.
3. Le Boit PE, Burg G, Weedon D, Sarasin A : Pathology and genetics skin tumors. OMS. 2006;1:131.
4. Busam KJ, Tan LK, Granter SR, Kohler S, Junkins-Hopkins J, Berwick M, et al: Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12:786-93.
5. Hiatt KM, Pillow JL, Smoller BR: Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod Pathol. 2004;17:28-32.
Comments are closed.