How narrow-band and broad-band uvb irradiation influences the histomorphology evaluations of experimental animals’ skin – a comparative study. Part I.
Katarzyna Borowska
Department of Histology and Embryology with Experimental Cytology Unit, Medical University of Lublin, 11 Radziwiłłowska, 20–080 Lublin, Poland
Corresponding author: Prof. Katarzyna Borowska, E-mail: k_borowska@wp.pl
Submission: 13.09.2017; Acceptance: 17.09.2017
How to cite this article: Borowska K. How narrow-band and broad-band uvb irradiation influences the histomorphology evaluations of experimental animals’ skin – a comparative study. Part I. Our Dermatol Online. 2017;8(3e):e5.
ABSTRACT
UVB phototherapy is an common and effective method of treating many skin diseases. The broad-band ultraviolet B (BB-UVB) spectrum is 290-320 nm. An alternative to this therapy is using the narrow-band ultraviolet B (NB-UVB). The aim of the study was to evaluate comparatively the influence of NB-UVB and BB-UVB phototherapy on the histomorphology (HM) of the skin of experimental animals. Both NB-UVB and BB-UVB phototherapy, result in microscopically observable changes in the epidermis and upper parts of the dermis papillary layer. No changes were observed, though, in the lower parts of the papillary layer or in the reticular layer of the dermis. The histological changes was more intense in the group exposed to BB-UVB radiation than in the NB-UVB group.
Key words: narrow-band UVB, TL01, 311 nm, broad-band UVB, TL 12.
INTRODUCTION
UVB phototherapy, i.e. treatment with the use of artificial sources UVB radiation is an common and effective method of treating many skin diseases for example mainly psoriasis [1], vitiligo [2], atopic eczema [3] and many others. The broad-band ultraviolet B (BB-UVB) spectrum is 290-320 nm and is emitted by TL12 lamps. An alternative to this therapy is using the narrow-band ultraviolet B (NB-UVB). Sometimes the name ‘311 nm’ is used too because the NB-UVB spectrum is very narrow indeed as it is 311-313 nm only. This type of radiation is emitted by TL01 lamps. The aim of the study was to evaluate comparatively the influence of NB-UVB and BB-UVB phototherapy on the histomorphology (HM) of the skin of experimental animals. In order to perform a comparative analysis of the UVB wavebands described above, the following parameters were assessed: (A) the kind of cellular changes and architectonics distortions in consecutive layers of the epithelium; (B) basement membrane continuity; (C) possible cellular infiltrations; and (D) damage and/or degeneration of connective tissue fibers including dermis.
MATERIAL AND METHODS
The above-described histological assessment was performed on two experimental groups. One – exposed to NB-UVB (311nm); the other – exposed to BB-UVB radiation (290-320 nm). The comparative analysis of how UVB radiation (both NB-UVB and BB-UVB) influenced the skin structure elements itemized above, was carried out in the irradiated skin versus the adjacent skin which was not irradiated. The experimental animals were white Wistar female rats weighing 250-300 g, from Lab Animals Rearing (Hodowla Zwierząt Laboratoryjnych) in Warsaw. The permission to use experimental animals in the study was granted by the 1st Local Ethics Committee for Animal Experiments. The female rats were randomly assigned to particular experimental groups and control group. The animals were placed in cages of 0.5 m2 each, in a room with the natural light cycle, temperature around 20ºC, and 60% humidity. The procedures were performed on animals immobilized on a table. During a test, the influence of stressful factors was maximally limited. The experimental animals and those from the control group were fed with granular food and water ad libitum. The sawdust litter was exchanged sufficiently frequently. All animals were killed by decapitation at the same time, 24 hours after the last irradiation.
The control group comprised of five female white Wistar rats. The animals were not exposed to UVB radiation, were given only food and water. The material to the study in the form of a 1-cm2 skin specimen was acquired from the central part of the dorsal region.
Experimental group – NB-UVB (NB-UVB-D and NB-UVB-A)
The NB-UVB experimental group consisted of 10 female white Wistar rats. The skin of these animals was exposed to NB-UVB radiation, i.e. the band of 311 nm. The site irradiated was the dorsal region. The part to be irradiated was shaved clean with a standard disposable razor. The hairs in the site were regularly shaved off during the period of the experiment. The irradiation procedures were performed once a day, always at the same time, five days a week (Monday through Friday) up to the total number of 20 irradiations. The light source was a lamp with narrow UVB waveband i.e. 311 nm (TL01), called TH-1 (Skintest-Kit by Cosmedico Medizintechnik). Before the experiment, the radiation intensity was measured. The intensity value at direct contact was 18.5 mW/cm2. Owing to a Skintest software by Cosmedico, once the radiation intensity was known, individual irradiation doses were calculated for particular irradiation times. During the experiment, the intensity of radiation was monitored. It did not change. The irradiation was performed at direct contact to the site on the smooth skin of the dorsal region, where the hairs had been shaved off. The time elapsed between shaving off hairs and irradiation was around two hours. Since the animal skin’s pigmentation was practically lacking, physical doses of NB-UVB radiation used in the experiment were comparable with those used in treating skin diseases in patients with the 1st Fitzpatrick phototype [4,5]. The scheme of irradiations in this experimental group was like clinical use in patients with the 1st Fitzpatrick phototype as follows:
Experimental group – BB-UVB (BB-UVB-D and BB-UVB-A)
The BB-UVB experimental group consisted of 10 female white Wistar rats. The scheme of the experiment in this study group was similar to that of the NB-UVB experimental group, but for one difference that in this group the skin of experimental animals was irradiated with BB-UVB, i.e. a band of 290-320 nm. The site irradiated was the dorsal region, which had been shaved clean. The irradiation procedures were performed once a day, always at the same time, five days a week (Monday through Friday) up to the total number of 20 irradiations. The intensity measured of the lamp BB-UVB was 17.0 mW/cm2. By knowing this value, it was possible to calculate individual irradiation doses for particular irradiation times. Physical doses of radiation used in the experiment, also in this experimental group, were comparable with those used in treating skin diseases in patients with the 1st Fitzpatrick phototype [4,5]. Also in the case of this lamp, the radiation intensity did not change. The scheme of irradiations in this experimental group was like clinical use in patients with the 1st Fitzpatrick phototype as follows:
The material for the study in the form of 1-cm² healthy skin specimens from the irradiated with BB-UVB central part of the dorsal region (BB-UVB-D experimental group), and from not irradiated lateral part of the abdominal region (BB-UVB-A experimental group).
The specimens underwent a histomorphologic examination. The specimens of full-thickness healthy skin, acquired both from the controls and experimental groups (NB-UVB-D, NB-UVB-A, BB-UVB-D, BB-UVB-A), underwent histomorphologic examinations in light microscope. Before being examined, the specimens were fixed in a chemical fixative – a 4% solution of buffer formalin (pH 7). After fixation, the material was dehydrated in an ethyl alcohol sequence of increasing concentrations. After the skin specimens were embedded in acetone and xylene, they were embedded in Histosec paraffin (Merck) and paraffin blocks were made. A rotary microtome by Leica (RM 2135) was used to slice the paraffin blocks. Having been cut into slices 6 µm thick, they were placed on basic glasses for tissue and cell structures to be contrasted, and then stained with hematoxylin and eosin (H+E) acc. to Masson, and with PAS (Periodic Acid Schiff) to identify carbohydrates. In order to do that, the paraffin slices were deprived of paraffin by being embedded in xylene, and then hydrated by being submerged in a sequence of ethyl alcohol solutions of decreasing concentrations, ending up in pure water. In the routine H+E staining, hematoxylin acc. to Mayer and eosin in alcohol solution were used. In staining acc. to Masson’s method, the following reagents were used: mixture of acidic fuxine, 1% phosphomolybdic acid, light green, and 5% phosphotungstic acid. In staining based on the PAS reaction, periodic acid and Schiff’s reagent were used. After the specimens were stained and rinsed, they were embedded in natural resin – Canadian balm. The staining mentioned above was applied to both control and experimental specimens: NB-UVB-D, NB-UVB-A, BB-UVB-D, BB-UVB-A. They were examined in light microscope and photographed with a Nicon camera with a digital adapter.
RESULTS
Control group
Staining: H+E, acc. to Masson, PAS reaction.
Experimental group – NB-UVB-D
H+E staining
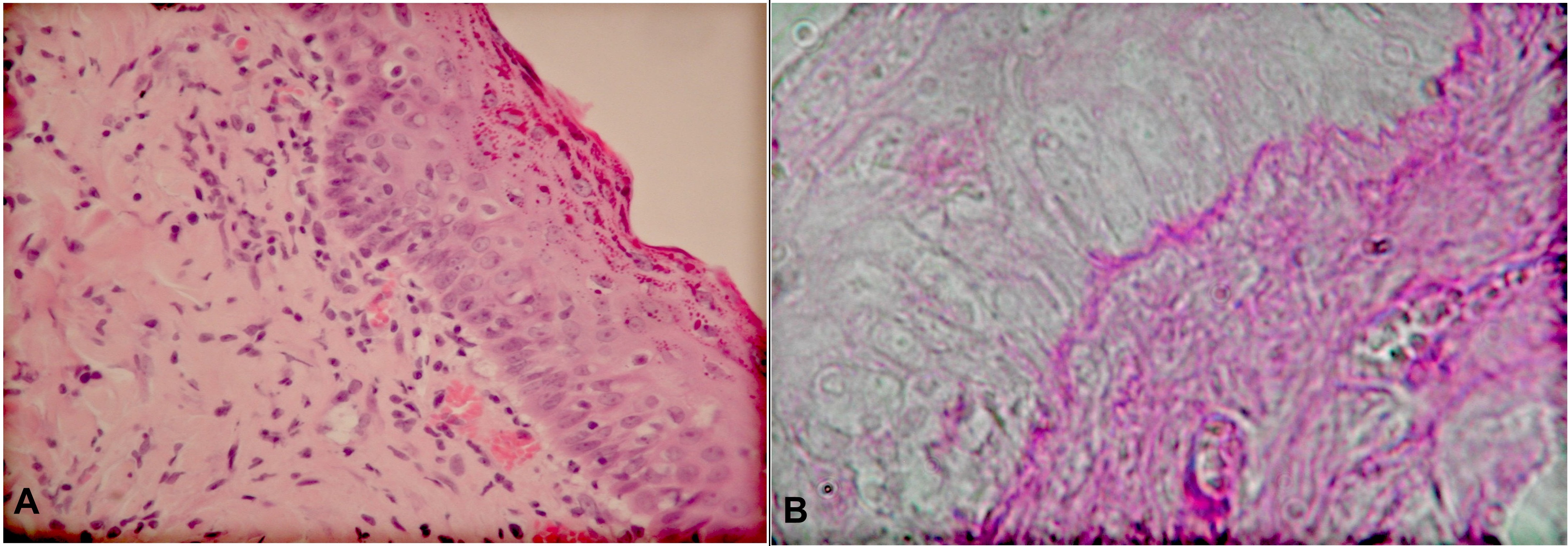
Epidermis histomorphology showed hypertrophy in comparison to the controls. Basal layer cells constituted one base and adhered to one another. Apart from cells with normal structure, there were such which looked like ‘small nails’. They were squeezed in between normal cells of the basallayer. Their cytoplasm was denser and their nuclei were slender. Part of keratinocyte nuclei of the basal layer had a clear chromatin stroma. There singular cells with division figures present. The examination of histological specimens towards the skin surface revealed hypertrophy of consecutive layers of the epidermis: spinous, granular and corneous. The spinous layer consisted of 3-5 layers of cells. The cells there were of different shapes, and the cytoplasm in different cells showed different affinity to acidic stains. There were singular cells with two nuclei. Most nuclei were low in chromatin. The cells in the next, granular, layer were flattened, their nuclei were low in chromatin. There were different amounts of keratohialin in their cytoplasm, which stained bright red. Also the corneous layer was wider and stained red. The dermal-epidermal border was maintained. Microscopic examination of the papillary layer of the dermis revealed infiltrates with mononuclear cells (lymphocytes, histiocytes) right beneath the epidermis. Such changes were absent from the skin specimens taken from control animals (Fig. 1a).
Staining acc. to Masson’s method
In the dermis, staining acc. to Masson’s method revealed connective tissue fibers, stained blue, organized into delicate bundles. In some of these bundles, their continuity was disrupted. Most fibers within the papillary layer of the dermis lay parallelly to the basement membrane. Their organization within the reticular layer was multidirectional. Connective tissue fibers surrounded sebaceous gland sacs.
PAS reaction
A positive PAS reaction revealed the basement membrane of the epidermis in the skin of the experimental animals’ NB-UVB-D group. There were isolated places, where the dermal-epidermal border was less distinct and looked ‘a little wavy’ (Fig. 1b).
a. H+E staining. Magn. ca 400x Hypertrophy of spinous, granular and corneous layers of epidermis. Inflammatory infiltration in upper parts of dermal papillary layer. b. PAS reaction. Magn. ca 800x. Dermal-epidermal border.
Experimental group – NB-UVB-A
The skin of the animals from this experimental group stained with H+E, acc. to Masson’s method, the PAS reaction. Their appearance was similar to that in the control group animals.
Experimental group – BB-UVB-D
H+E staining
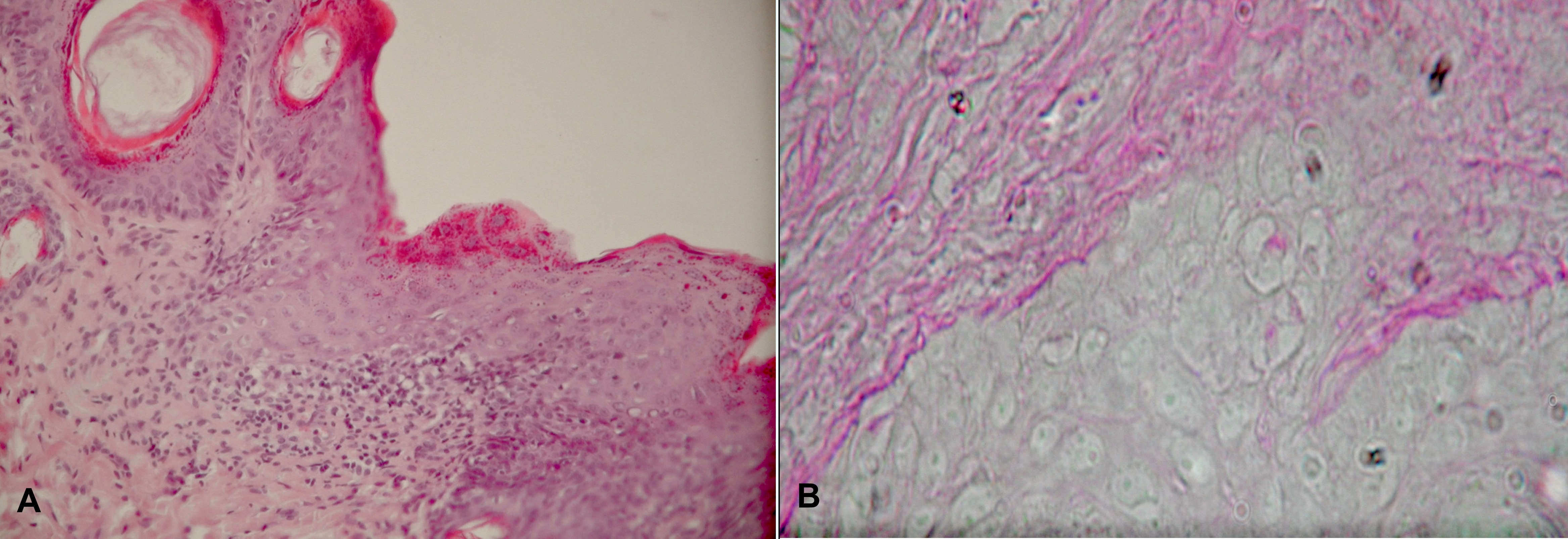
Unlike to what was observed in the control group, in this experimental group the epidermis of the dorsal region stained with H+E was hypertrophic. In the hypertrophic epidermis, especially in places where the dermal-epidermal border was indistinct, the architectonics of the epidermis was distorted. In infiltrated places, keratinocytes did not reveal the stratified structure so characteristic of the epidermis. Their organization was random. There were places, where the basal cell layer was very distinct, and the cells maintained their palisade pattern. In the remaining regions there were observed narrow cells of ‘small nails’ appearance squeezed in between cells with normal histological structure. The spinous layer consisted of several layers of cells, oval or circular. The cytoplasm of these cells had affinity of different degree to acidic stains. The nuclei in these cells had one or two nucleoli. The granular layer consisted of flattened cells containing stained granules of keratohialin in their cytoplasm. This layer was wider than its counterpart in the controls; it was similar to that in the NB-UVB-D experimental group. In fragments, the dermal-epidermal border was disrupted. At this border and in the upper regions of the papillary layer in the dermis there were streaky infiltrations of mononuclear cells – lymphocytes and histiocytes. The infiltrations were more intense than those observed in the NB-UVB-D experimental group. Apart from the infiltrations in the papillary layer, the microscopic examination did not reveal any other significant abnormalities in the dermis histomorphology (Fig. 2a).
Staining acc. to Masson’s method
Staining acc. to Masson’s method revealed connective tissue fibers which stained blue. In the papillary layer, the fibers lay parallelly to the basement membrane; their pattern in the reticular layer was multidirectional. In comparison to the controls, connective tissue fibers in the papillary layer were organized more loosely. In the dermis, staining acc. to Masson’s method revealed connective tissue fibers, stained blue, organized into delicate bundles. Some of these bundles were disrupted, their continuity was disrupted. These disruptions were greater than the ones observed in the NB-UVB-D group. In some places, the dermal-epidermal border was indistinct, and there were infiltrations with mononuclear cells parallelly to the epidermis. Apart from that, there were no significant histological changes within the dermis. The corneous layer was very distinct and hypertrophic; it stained bright red. Microscopically, the architectonics of the epidermis was distorted in parts, especially where there was an infiltration of mononuclear cells.
PAS reaction
The hypertrophic epidermis with partially distorted architectonics lay on a little wavy basement membrane, which in PAS reaction was positive. There were places where the basement membrane stained dark red was clearly disrupted (Fig. 2b).
a. H+E staining. Magn. ca 400x. Hypertrophic epidermis with partially invisible dermal-epidermal border and infiltrations in upper parts of dermal papillary layer. b. PAS reaction. Magn. ca 800x. Disrupted continuity of dermal-epidermal border.
Experimental group – BB-UVB-A
The skin of the animals from this experimental group stained with H+E, acc. to Masson’s method, the PAS reaction. Their appearance was similar to that in the control group animals.
DISCUSSION
The positive therapeutic outcome of using UVB (NB-UVB and BB-UVB) stimulates scientific research aiming at understanding the effects of these wavebands at the cellular and subcellular levels. Most microscopic studies carried out so far which compared the effect of NB-UVB and BB-UVB irradiation in the skin, have focused on the microscopic assessment of the skin mainly following singular exposures or studies of cellular lines in vitro [6-8]. The only study published where multiple NB-UVB and BB-UVB exposures are compared, describes using repetitive suberythema doses (0.3MED/0.35MED), in their total value much lower than those used routinely in phototherapy [9]. The present study analyzed histological changes in healthy epidermis and dermis of Wistar rats following multiple irradiations with increasing doses of NB-UVB and BB-UVB, used to treat patients with the 1st Fitzpatrick skin phototype [4,5]. The experiment also included analyzing a possible impact UVB radiation (NB-UVB and BB-UVB) had on the epithelium and connective tissue in the direct vicinity of an irradiated site (NB-UVB-A and BB-UVB-A experimental groups). So far, there have been no data in literature describing this type of histological analysis. The present experiment proved that multiple increasing doses of UVB radiation, both NB-UVB and BB-UVB, result in microscopically observable changes in the epidermis and upper parts of the dermis papillary layer. No changes were observed, though, in the lower parts of the papillary layer or in the reticular layer of the dermis. The analysis in light microscope of specimens from NB-UVB-D and BB-UVB-D experimental groups revealed hypertrophy of consecutive epidermis layers: spinous, granular and corneous. Epidermal hypertrophy was more pronounced in the group exposed to BB-UVB radiation (290-320 nm) than in the NB-UVB-D group. Persistent irradiation of the dorsal region in C3H/Hen mice with much lower doses of NB-UVB and BB-UVB than those use in the present study, did not cause epidermal hypertrophy in NB-UVB irradiated mice, but there was a 50% increase of the epidermis thickness observed in those exposed to BB-UVB. Exposures with repetitive and suberythema doses were performed three times a week for weeks. In the case of NB-UVB, the constant dose was 0.3 J/cm2 until it reached the total of 5.4 J/cm2. With BB-UVB, the dose was 0.05 J/cm2, and total = 0.9 J/cm2 [9]. The discrepancies in the studies on NB-UVB influence on the epidermal hypertrophy presented above may results from different suberythema NB-UVB doses and different irradiation patterns. In the study with C3H/Hen mice the doses used were multiple but repetitive. In the present study, Wistar rats were exposed to repetitive doses, and the cumulative dose was much higher. With NB-UVB, it was 3.54 J/cm², with BB-UVB – 17.18 J/cm². Sometimes consistent results of studies may not reflect the differences resulting from differences among experimental animals (white Wistar rats) and different strains of mice (Skh-hr 1, C3H/Hen). The results obtained in the present study confirm the supposition that persistent irradiation with increasing doses used in phototherapy provokes the epidermis to overgrow after exposure to both NB-UVB and BB-UVB. The present study confirms earlier observations that BB-UVB induces epidermal overgrowth to a larger extent than NB-UVB does. Berton et al. showed in their study that increased epidermal proliferation correlates with neoplasm induction [10]. Consequently, epidermal overgrowth can be suspected of being an indirect factor for increased risk of photocarcinogenesis. BB-UVB more than NB-UVB, having a bigger epidermal hypertrophy potential, can also be suspected of increased risk for triggering a neoplastic process. In particular segments of hypertrophic epidermis in the experimental groups (NB-UVB-D and BB-UVB-D), along with normal cells, there were elongated cells somehow ‘squeezed’ in between unchanged cells of the basal layer. These cells had slender nuclei and denser cytoplasm. Microscopically, in the both groups there were singular cells of the basal layer, which contained division figures illustrating numerous mitotic divisions in the generative layer. In the specimens obtained from the regions in close vicinity of an irradiated site (NB-UVB-A and BB-UVB-A experimental groups) no epidermal hypertrophy was observed. Epidermal histomorphology in the two groups was similar to that in the controls. In the BB-UVB-D experimental group, besides places with distinct palisade and regular pattern of the basal layer cells in the hypertrophic epidermis, there were places with unclear dermal-epidermal border. In some places, this border was clearly disrupted. There are studies indicating that a damaged basement membrane is an early indicator of neoplastic process progression [11-13]. In the case of skin carcinoma in situ (Bowen’s disease, erythroplasia of Queyrat) undamaged membrane is a barrier to further proliferation of neoplastic cells. In places where the dermal- epidermal barrier was damaged, the architectonics of the epidermis was distorted. The pattern of keratinocytes was random and histologically absolutely unlike that in the controls. A damaged basement membrane allows fibroblasts to interact with keratinocytes, thus inducing the following proliferation factors to act: growth factor, cytokines, dermal intercellular matrix proteases. These factors cause epidermal proliferation to increase [14]. The PAS reaction proved the continuity of the dermal-epidermal border in the NB-UVB-D experimental group. Microscopically, though, this border was less clear and had some ‘wavy’ features. In the specimens from the groups not irradiated (NB-UVB-A and BB-UVB-A groups) just like in the control group, the border was very distinct. The BB-UVB radiation (BB-UVB-D experimental group), apart from disrupting dermal-epidermal border and distorting the cellular architectonics, resulted in an mononuclear cell infiltration – lymphocytes and histiocytes. The infiltrations were mostly found parallelly to the epidermis on the dermal-epidermal border and in the upper parts of the papillary layer in the dermis. In the places affected by the infiltrations, the dermal-epidermal border was visibly disrupted, and the epidermis over the site did not have the stratified structure so characteristic of the epidermis. Its architectonics was distorted. Similar infiltrations, but much less pronounced, were observed beneath the epidermis in the experimental group exposed to narrow-band UVB radiation. A larger infiltration observed in the group exposed to BB-UVB can be explained hypothetically by a quicker infiltration formation as a result of BB-UVB induction or a slower elimination of the infiltration during apoptosis.
The fact that in psoriasis and other inflammatory diseases BB-UVB is less effective a compared with NB-UVB is connected with a weaker elimination of the inflammatory process. Persistent inflammation and inflammatory infiltrations also predispose to a neoplastic process. Studies using COX 2 (cyclooxygenase 2) inhibitors and animals (COX-2 knockout mice) proved that COX 2 induction is responsible for inflammatory infiltration formation, edema, keratinocyte proliferation, epidermal hypertrophy, and free radicals that initiate DNA damage. Chronic skin inflammation and increased COX 2 activity together with the accumulation of DNA damage and mutations may trigger skin cancer development. Inhibiting COX 2 activity significantly decreases carcinogenesis induced by persistent UVB irradiation [15,16]. Also clinically, many diseases associated with chronic inflammation may lead to skin cancer, e.g. discoid lupus erythematosus, dystrophic epidermolysis bullosa. On the other hand, in many other skin disease involving chronic inflammation, such as psoriasis, atopic dermatitis, Darier disease, higher cancer morbidity rate is not observed. Besides, the activation of an inflammatory process is connected with the release of cytokines, growth factors, prostaglandins, tumor necrosis factor-alpha (TNF-alpha), AP-1, nuclear factor-kB (NF-kB), which participate in inducing cancer by UV radiation. The induction of the NF-kB transcription factor is partially responsible for recruiting inflammatory cells from the skin circulation. This factor was found to influence the regulation of post-inflammatory cytokine expression – IL-1β (interleukin 1β), TNF (tumor necrosis factor) [17]. The findings and results of the present study confirm that UV radiation contributes to triggering mechanisms of immune response. The consequence of how this radiation works is skin infiltration by inflammatory cells [18]. It has been pointed out lately that UVB radiation may react to infiltrate cell formation in the dermis by inducing cytokines in keratinocytes. Microscopic observations during the study confirmed that UVB radiation, both NB-UVB and BB-UVB, induces mononuclear infiltration formation (with lymphocytes and histiocytes). Infiltrations were not observed in the places adjacent to the site of UVB irradiation (NB-UVB-A and BB-UVB-A experimental groups). Consequently, it can be said that UVB radiation, either NB-UVB and BB-UVB, does not affect infiltration formation in the skin directly adjacent to the site of UVB exposure, but unaffected itself. Changes in the dermis were also analyzed in the study. In the upper parts of the papillary layer, in numerous fiber bundles were disrupted, which resulted in the breaking of their continuity. Connective tissue fibers were organized more loosely in the upper parts of the papillary layer than what was seen in the controls. Such changes were observed in the both experimental groups (NB-UVB-D and BB-UVB-D), yet they were more pronounced in the group exposed to the BB-UVB radiation. An inflammatory infiltrate leads also to edema formation and induces many enzymes participating in the degradation of the extracellular matrix and, possibly, connective tissue fibers. A looser pattern of fibers in the upper parts of the papillary layer might be explained by a streak-like pattern of inflammatory infiltrates. This hypothesis explains a looser pattern of connective tissue fibers in the BB-UVB-D experimental group, where infiltrates are more pronounced than in the NB-UVB-D group. In the histological examination, the lower parts of the papillary layer and reticular layer were similar to those in the controls. This confirms a more superficial action of UVB in comparison with a more penetrating UVA radiation of a longer waveband (320-400 nm). The data obtained after the results were analyzed allowed for a clinically important comparative assessment of the two wavebands of radiation. Consequently, they may result in a more rational (than just empirical) application of UVB phototherapy, and contribute to a safer treatment of skin diseases.
In conclusions, both NB-UVB and BB-UVB phototherapy, result in microscopically observable changes in the epidermis and upper parts of the dermis papillary layer. No changes were observed, though, in the lower parts of the papillary layer or in the reticular layer of the dermis. The histological changes was more intense in the group exposed to BB-UVB radiation than in the NB-UVB group.
REFERENCES
1. Levin AA, Aleissa S, Dumont N, Martinez F, Donovan C, Au SC, et al. A randomized, prospective, sham-controlled study of localized narrow-band UVB phototherapy in the treatment of plaque psoriasis. J Drugs Dermatol. 2014;13:922-6.
2. Li R, Qiao M, Wang X, Zhao X, Sun Q. Effect of narrow band ultraviolet B phototherapy as monotherapy or combination therapy for vitiligo: a meta-analysis. Photodermatol Photoimmunol Photomed. 2017;33:22-31.
3. Reynolds NJ, Franklin V, Gray JC, Diffey BL, Farr PM. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet. 2001;357:2012-6.
4. Fitzpatrick T. Soleil et peau. J Med Esthet. 1975;2:33–4.
5. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of dermatology. 1988;124:869–71.
6. Aufiero BM, Talwar H, Young C, Krishnan M, Hatfield JS, Lee HK, et al. Narrow-band UVB induces apoptosis in human keratinocytes. J Photochem Photobiol B. 2006;82:132-9.
7. Xu S, Li L, Li M, Zhang M, Ju M, Chen X, et al. Impact on Autophagy and Ultraviolet B Induced Responses of Treatment with the MTOR Inhibitors Rapamycin, Everolimus, Torin 1, and pp242 in Human Keratinocytes.Oxid Med Cell Longev. 2017;2017:5930639.
8. He J, Long C, Huang Z, Zhou X, Kuang X, Liu L, et al. PTEN Reduced UVB-Mediated Apoptosis in Retinal Pigment Epithelium Cells. Biomed Res Int. 2017;2017:3681707.
9. el-Ghorr AA, Norval M, Lappin MB, Crosby JC. The effect of chronic low-dose UVB radiation on Langerhans cells, sunburn cells, urocanic acid isomers, contact hypersensitivity and serum immunoglobulins in mice. Photochem Photobiol. 1995;62:326-32.
10. Berton TR, Mitchell DL, Fischer SM, Locniskar MF. Epidermal proliferation but not quantity of DNA photodamage is correlated with UV-induced Morse skin carcinogenesis. J Invest Dermatol. 1997;109:340.
11. Bassi DE, Lopez De Cicco R, Cenna J, Litwin S, Cukierman E, et al. PACE4 expression in mouse basal keratinocytes results in basement membrane disruption and acceleration of tumor progression. Cancer Res. 2005;65:7310-9.
12. Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67-8.
13. Tennenbaum T, Yuspa SH, Grover A, Castronovo V, Sobel ME, Yamada Y, et al. Extracellular matrix receptors and mouse skin carcinogenesis: altered expression linked to appearance of early markers of tumor progression. Cancer Res. 1992;52):2966-76.
14. Mueller MM, Fusenig NE. Friends or foes-bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839-49.
15. Fischer SM, Pavone A, Mikulec C, Langenbach R, Rundhaug JE. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol Carcinog. 2007;46:363-71.
16. Rundhaug JE, Mikulec C, Pavone A, Fischer SM. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 2007;46:692-8.
17. Kang S, Fisher GJ, Voorhees JJ. Photoaging: pathogenesis, prevention, and treatment. Clin Geriatr Med. 2001;17:643-59.
18. Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y, Datta S, Wang ZQ, et al. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J Invest Dermatol. 2001;117:219-26.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Comments are closed.