Eosinophilic fasciitis: Case report
Maha Lahouel 1, Yosra Soua1, Leila Njim2, Ines Lahouel1, Malek Kechida3, Hichem Belhadjali1, Monia Youssef1, Jameleddine Zili1
1, Yosra Soua1, Leila Njim2, Ines Lahouel1, Malek Kechida3, Hichem Belhadjali1, Monia Youssef1, Jameleddine Zili1
1Department of Dermatology, Fattouma Bourguiba Hospital, Monastir, Tunisia:2Department of Pathology, Fattouma Bourguiba Hospital, Monastir, Tunisia3Department of Internal Medicine, Fattouma Bourguiba Hospital, Monastir, Tunisia
Corresponding author: Dr. Maha Lahouel
Submission: 14.11.2019; Acceptance: 15.01.2020
DOI: 10.7241/ourd.2020e.91
Cite this article: Lahouel M, Soua Y, Njim L, Lahouel I, Kechida M, Belhadjali H, Youssef M, Zili J. Eosinophilic fasciitis: Case report. Our Dermatol Online. 2020;11(e):e91.1-e91.3.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Eosinophilic fasciitis or Shulman’s disease is a rare connective tissue disorder that has variable presentations. We report the case of an elderly woman who presented with a 4 months’ history of skin induration of forearms and legs following edema of the extremities. Physical examination revealed the presence of sclerotic plaque infiltrating the subcutaneous tissue. Skin and muscle biopsy in the leg was in favor of a shulman fasciitis. MRI showed an inflammatory involvement of the fascia. Boreal serology was positive Ig M-type twice at 15-day intervals. The patient was placed on amoxicillin with the occurrence of a Herxheimer reaction. The diagnosis of eosinophilic fasciitis associated with a borrelin infection was retained. The patient has shown significant improvement under oral corticosteroids associated with methotrexate. Shulman fasciitis can pose great diagnostic difficulty in the elderly. Its possible association with Lyme disease is discussed.
Key words: Eosinophilic fasciitis; Shulman disease; Borrelia, Lyme disease
INTRODUCTION
Eosinophilic fasciitis (EF) or Shulman’s disease is a rare connective tissue disorder characterized by symmetrical and painful swelling followed by progressive induration and thickening of the underlying soft tissue and skin [1,2]. We report a new case of eosinophilic fasciitis and discuss the relationship with Lyme borreliosis.
CASE REPORT
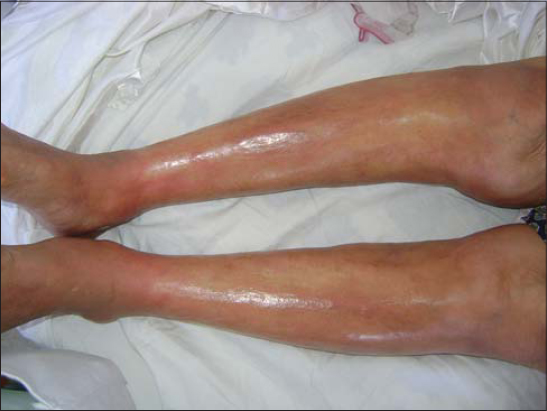
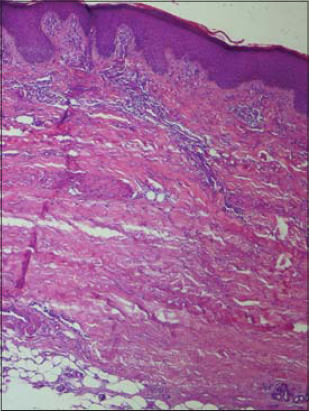
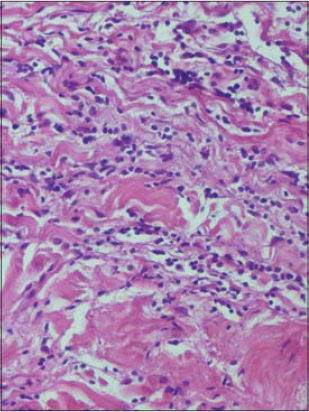
An 82-year-old woman consulted for a skin induration of the forearms and legs that had evolved for 1 month following edema of the extremities without the notion of a tick bite, travel in a forest zone, or taking medication. Physical examination revealed the presence of sclerous skin of the forearms and legs (Fig. 1). The patient presented also with indurated peau-d’orange-like skin on the legs (Fig. 2). A grooving of the skin between muscles and sunken veins was noted. There was no involvement of the face, sclerodactyly, or Raynaud’s syndrome. The rest of physical examination was without particularities. The eosinophilic polynuclear count was 500 elements/µl. Polyclonal hypergamapathy was noted. Skin and muscular biopsy of the leg was in favor of a shulman fasciitis showing perivascular inflammatory infiltrates composed of lymphocytes and eosinophils, without associated vasculitis (Figs. 3 and 4). MRI showed an inflammatory involvement of the fascia. Radiological investigations eliminated underlying neoplasia. IgM western blot were positive. Autoimmune screening for ANCA and anti-DNA/ENA antibodies is negative.
In view of these clinical and histological signs, we have carried the diagnosis of eosinophilic fasciitis associated with a probable borrelin infection. The patient was placed on amoxicillin. a pruriginous exanthema appeared on the 5th day. Amoxicillin was replaced by doxycycline. Additional treatment with oral corticosteroids at a dose of 1 mg/kg/day associated with methotrexate was instituted, with partial improvement.
DISCUSSION
Eosinophilic fasciitis is a rare connective tissue disorder. It was first described by Lawrence Shulman in 1974 [3]. Since then, over 300 cases have been reported worldwide [2]. EF has variable clinical presentations. Characteristic findings include scleroderma-like skin changes. The onset of EF is marked by acute and subacute erythematous swelling of the affected extremities. As the disease progresses, edema is gradually replaced by a ‘peau d’orange’ aspect as deep sclerosis starts to develop. The Groove sign is another characteristic feature consisting of a linear depression in the skin parallel to the course of the superficial veins and, more marked on elevation of the affected limb [2]. Extremities are affected symmetrically, with the exclusion of hands and feet. Neck and truncal involvement are reported in widespread disease [1]. Cutaneous involvement may be accompanied by general symptoms such as weight loss, myalgia, and asthenia. The lack of Raynaud’s phenomenon, telangiectasia, and visceral involvement differentiate it from Scleroderma [4,5]. Peripheral eosinophilia and eosinophilic infiltration of the fascia are not obligatory to establish the diagnosis [4]. Hypereosinophilia is not constant (60-90%) [6]. Extracutaneous manifestations include arthralgia, arthritis, joint contracture and carpal tunnels.
According to criteria proposed by Pinal-Fernandez et al. in 2014, our patient filled two major criteria and two minor criteria, as well as the exclusion of Systemic Scleroderma.
Major criteria are (a) Symmetric diffuse induration on the extremities and full-thickness wedge biopsy of clinically affected skin showing fascial thickening with accumulation of lymphocytes and macrophages with or without eosinophils. Minor criteria are peau d’orange-appearing skin and T2-weighted MRI showing hyperintense fascia.
The biopsy must include the fascia. It showed a thickening of the fascia caused by lymphocyte and plasma cell infiltration with a varying percentage of eosinophil granulocytes. Eosinophilic infiltration is only seen in the early stages of the disease.
Magnetic resonance imaging is indicated to locate a suitable biopsy site and to monitor treatment response. It shows an increased T2 signal in subcutaneous and deep fascia.
Eosinophilic fasciitis can be associated with hematological disorders in nearly 10% of patients and exceptionally with solid tumors or autoimmune diseases [7]. The search for hemopathy or neoplasia in our patient was negative.
The exact pathogenesis remains unclear. Most cases are idiopathic. Some cases are associated with strenuous exercise [8], trauma [7], initiation of hemodialysis, radiation therapy, hematologic disorders, Graft-versus-host disease and infection with Borrelia burgdorferi [9–13]. Borrelia origin has been implicated in the etiopathogenesis of eosinophilic fasciitis, as some authors reported the notion of tick bite before the occurrence of fasciitis and detection of the spirochete by PCR in these patients [14]. The positivity of Borrelia serology in our patient twice, the occurrence of Herxheimer reaction are arguments in favor of Borrelia infection.
The therapeutic management of eosinophilic fasciitis associated with Lyme disease is not coded [5]. Oral corticosteroids, with or without methylprednisolone pluses, remain the main treatment with a significant improvement for the majority of patients [4]. It might be associated with methotrexate, in patients with an unsatisfactory response to corticosteroids alone, as in our case.
CONCLUSION
Shulman fasciitis is evoked in the front of sclerous and indurated peau d’orange like skin. It must be differentiated from systemic scleroderma. Its possible association with Lyme disease is discussed and pose a therapeutic problem.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
REFERENCES
1. Sene D. [Eosinophilic fasciitis (Shulman’s disease):Diagnostic and therapeutic review]. La Revue Med Inter. 2015;36:738-45.
2. Fett N, Arthur M. Eosinophilic fasciitis:Current concepts. Clin Dermatol. 2018;36:487-97.
3. Shulman LE. |iDiffuse fasciitis with hypergammaglobulinemia and eosinophilia:a new syndrome?J Rheumatol. 1984;11:569-70.
4. Jinnin M, Yamamoto T, Asano Y, Ishikawa O, Sato S, Takehara K, et al. Diagnostic criteria, severity classification and guidelines of eosinophilic fasciitis. J Dermatol. 2018;45:881-90.
5. Mazori DR, Femia AN, Vleugels RA. Eosinophilic Fasciitis:an Updated Review on Diagnosis and Treatment. Curr Rheumatol Rep. 2017;19:74.
6. Ernest V, Sautereau N, Masson E, Chemouni D, Garcia M, Bertolino J, et al. [Eosinophilia heralding the diagnosis of eosinophilic fasciitis (Shulman’s disease)]. Rev Med Inter. 2017;38:840-3.
7. Pinal-Fernandez I, Selva-O’Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev. 2014;13:379-82.
8. Wright NA, Mazori DR, Patel M, Merola JF, Femia AN, Vleugels RA. Epidemiology and Treatment of Eosinophilic Fasciitis:An Analysis of 63 Patients From 3 Tertiary Care Centers. JAMA Dermatol. 2016;152:97-9.
9. Raveendra L, Raju BP, Nagaraju U, Vivekananda, Sundar PK, Keshavalu L. Atrophic type of morphea profundus – an Indian experience. Our Dermatol Online 2013;4:172-5.
10. Yazdanpanah MJ, Sharifi N, Khooei A, Banihashemi M, Khaje-Daluee M, Shamsi A, Ghazvini K. Frequency of borrelia in morphea lesion by polymerase chain reaction in northeast of Iran. Jundishapur J Microbiol. 2015;8:e19730.
11. Mertens JS, Seyger MMB, Thurlings RM, Radstake TRDJ, de Jong EMGJ. Morphea and eosinophilic fasciitis:an update. Am J Clin Dermatol 2017;18:491-512.
12. Tolkki L, Hokynar K, Meri S, Panelius J, Puolakkainen M, Ranki A. Granuloma annulare and morphea:correlation with borrelia burgdorferi infections and chlamydia-related bacteria. Acta Derm Venereol. 2018;98:355-60.
13. Belot V, Mulleman D, Perrinaud A, Abdallah-Lotf M, Machet MC, Machet L. [Eosinophilic fasciitis associated with Borreliaburgdorferi infection]. Ann Dermatol Venereol. 2007;134:673-7.
14. George E, Nielson CB, Vincek V. Tick bite-associated morphea:A Case Report. Am J Dermatopathol. 2019;41:747-9.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/000-0003-3455-3810 http://orcid.org/000-0003-3455-3810 |




Comments are closed.