Imatinib induced melasma-like pigmentation
Consultant Dermatologist, Treatwell Skin Centre, Jammu, India
Corresponding author: Dr. Mrinal Gupta
Submission: 01.03.2020; Acceptance: 06.04.2020
DOI: 10.7241/ourd.2020e.53
Cite this article: Gupta M. Imatinib induced melasma-like pigmentation. Our Dermatol Online. 2020;11(e):e53.1-e53.2
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Imatinib mesylate, a tyrosine kinase inhibitor, is used for the treatment of chronic myeloid leukemia (CML), gastrointestinal stromal tumor (GIST), mastocytosis, myelodysplastic and myeloproliferative disorders. Cutaneous side effects of imatinib include xerosis, photosensitivity, pigmentary changes hypopigmentation and rarely hyperpigmentation. A 19-years-old male diagnosed case of CML currently on Imatinib 400mg once daily for 18 months presented with gradually progressive brownish discoloration of face since 6 months. Based on history, clinical features and a temporal association with drug intake, a diagnosis of imatinib-induced melasma-like pigmentation was made and the patient was started on sunscreen and topical depigmenting agents.
Key words: Drug-induced pigmentation; Imatinib-induced pigmentation; Melasma like pigmentation
INTRODUCTION
Imatinib mesylate is a tyrosine kinase inhibitor that targets BCR-ABL tyrosine kinase, platelet derived 1 growth factor (PDGF), and c-kit. FDA approved indications of imatinib are Philadelphia chromosome positive adult chronic myeloid leukemia (CML), KIT positive metastatic gastrointestinal stromal tumor, acute myelogenous leukaemia, myelodysplastic/myeloproliferative diseases, hypereosinoplilic syndrome, aggressive mastocytosis and refractory dermatofibrosarcoma protuberans [1]. Various cutaneous adverse effects have been reported with Imatinib. Reversible hypopigmentation is a well-recognized side effect of this drug; however, hyperpigmentation like melasma has also been reported [2,3].
We report a case of Imatinib induced melasma-like hyperpigmentation in a 19-year old male, who had been on Imatinib treatment for 18 months.
CASE REPORT
A 19-year-old male, a diagnosed case of CML on treatment with Imatinib mesylate 400 mg daily since 18 months, presented with gradually progressive asymptomatic brownish discoloration of face for 6 months. Cutaneous examination revealed brownish hyperpigmented patches involving the malar areas of the face extending bilaterally to zygomatic areas and bridge of the nose (Figs. 1a and 1b). There was sparing of the upper and lower eyelids and the nasolabial folds. The palms, soles and nails were normal and the buccal mucosa and teeth were also not involved.
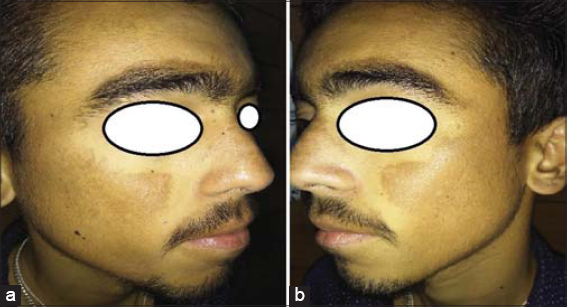 |
Figure 1: (a and b) Diffuse brownish pigmentation over cheek and nose. |
There was no history of excessive sunexposure or any topical application prior to the onset of hyperpigmentation. The patient was advised a skin biopsy, but it was refused by the parents of the patient. Based on history, clinical features and a temporal association with drug intake, a diagnosis of imatinib-induced melasma-like pigmentation was made and the patient was started on sunscreen and topical depigmenting agents.
DISCUSSION
Imatinib belongs to a new class of anti-cancer drugs that target the tyrosine kinase receptor. This agent blocks signaling via BCR-ABL, c-kit and platelet derived growth factor receptor by binding to the adenosine triphosphate-binding pocket which is required for phosphorylation and activation of the receptor, resulting in inhibition of tumor proliferation. The common side effects of imatinib include nausea, myalgia, maculopapular rash and edema. Cutaneous adverse effects of imatinib include xerosis, photosensitivity, pigmentary changes, psoriasiform rash, angular chelitis, lichenoid reaction, acute generalized exanthematous pustulosis and painful oral erosions [1–3].
Pigmentary changes have been commonly reported with Imatininb, which include hypopigmentation, hyperpigmentation, generalized skin lightening, vitiligo like lesions and hair graying [1]. Depigmentation with Imatininb has been attributed to the inhibition of c-kit and PDGF receptors, which play a pivotal role in the melanogenesis pathway [2]. The reason behind hyperpigmentation may be linked to alterations in the c-kit signaling pathway as in families carrying congenital tyrosine II domain mutations of c-kit, a similar cutaneous phenotypic expression has been observed [2]. Other reasons postulated behind hyperpigmentation include paradoxical stimulation of melanocytes, drug – induced basal cell degeneration and deposition of drug metabolite that chelates melanin [4]. Imatinib induced pigmentation has also been reported in palatal mucosa, nails, teeth, hair, gums [5].
As Imatinib induced pigmentation is reversible, it doesn’t warrant cessation of drug therapy and it can be managed with topical depigmenting agents and strict sunprotection. Patient should be well informed about this side effect to maintain the treatment compliance.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
REFERENCES
1. Ghunawat S, Sarkar R, Garg VK. Imatinib induced melasma – like pigmentation:Report of five cases and review of literature. Indian J Dermatol Venereol Leprol. 2016;82:409-12.
2. Balasubramanian P, Jagadeesan S, Thomas J. Imatinib-induced extensive hyperpigmentation in a case of chronic myeloid leukemia. Indian J Dermatol. 2015;60:523.
3. Balagula Y, Pulitzer MP, Maki RG, Myskowski PL. Pigmentary changes in a patient treated with imatinib. J Drugs Dermatol. 2011;10:10626.
4. Alexandrescu DT, Dasanu CA, Farzanmehr H, Kauffman L. Persistant cutaneous hyperpigmentation after tyrosine kinase inhibition with imatinib for GIST. Dermatol Online J. 2008;14:7.
5. Li CC, Malik SM, Blaeser BF, Dehni WJ, Kabani SP, Boyle N, et al. Mucosal pigmentation caused by imatinib:Report of three cases. Head Neck Pathol. 2012;6:290-5.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|




Comments are closed.