New H2O2 dermocosmetic in acne skin care
Katarzyna Kisiel1, Renata Dębowska2, Katarzyna Dzilińska1, Agnieszka Radzikowska2, Monika Pasikowska-Piwko2, Katarzyna Rogiewicz2, Irena Eris2
¹Department of Pediatric Dermatology, Center of Dermatology, Międzyleski Specialist Hospital, Bursztynowa 2, 04-749 Warsaw, Poland, ²Dr Irena Eris Cosmetic Laboratories
Corresponding author: Katarzyna Kisiel, MD., E-mail: kkilian@wp.pl
Submission: 15.05.2018; Acceptance: 26.05.2018
DOI: 10.7241/ourd.2018e.1
How to cite this article: Kisiel K, Dębowska R, Dzilińska K, Radzikowska A, Pasikowska-Piwko M, Rogiewicz K, Eris I. New H2O2 dermocosmetic in acne skin care. Our Dermatol Online. Our Dermatol Online. 2018;9(e):e3.
ABSTRACT
Background: Acne vulgaris (AV) is a common skin disease with characteristic clinical, chronic course and different etiopathogenesis. AV occurs more and more often among adults, especially women. AV, due to the chronic nature of the skin lesions, often requires many months or even long-term treatment and proper skin care both during the period that does not require administration of drugs, as well as during and after pharmacotherapy. The aim of the study was to evaluate the safety and the anti-acne efficacy of a point-gel containing 2% H2O2 and salicylic acid (0.54%), phytic acid (1%), D-panthenol (1%) and vitamin PP (0.012%).
Material and methods: 24 patients with mild to severe AV used the study product containing hydrogen peroxide for 7 days. The condition of skin patients was assessed instrumentally (VISIA) and clinically by a dermatologist, including assessment of skin lesions visibility in the 10-point analogue scale and the number of particular acne lesions.
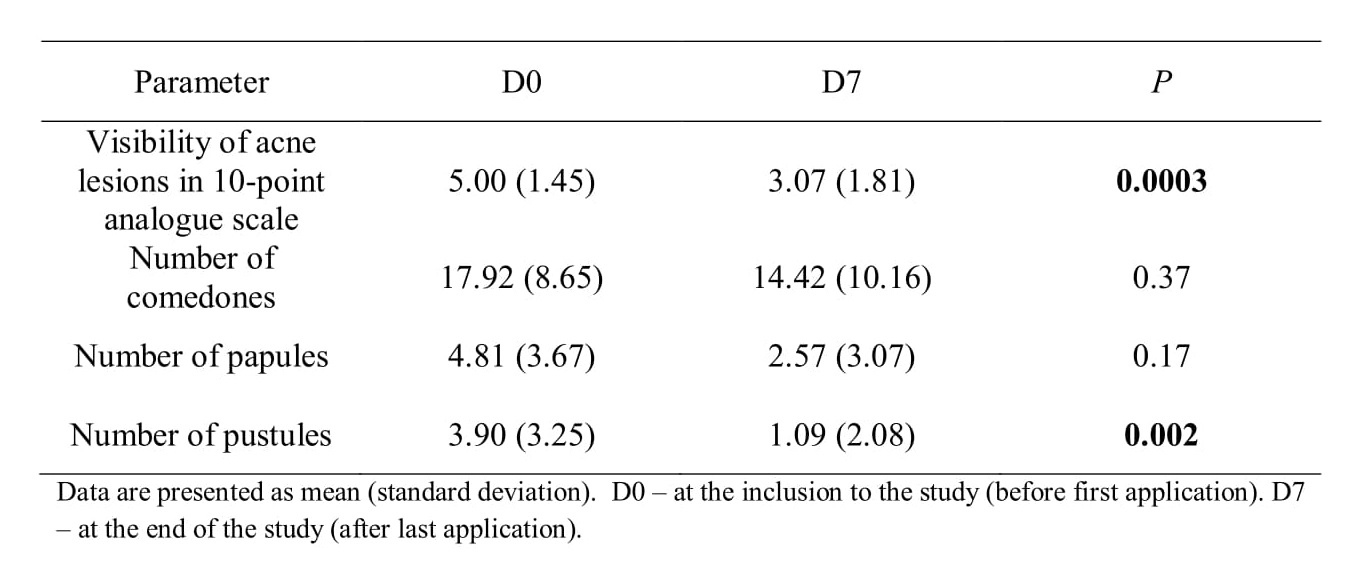
Results: The product was well tolerated. Dermatological evaluation at day 7 showed a significant decrease in the visibility of acne lesions (P=0.0003) and a significant reduction of number of pustules (P=0.002); an insignificant decrease in numbers of papules and comedones was also observed. Instrumental analysis of VISIA showed insignificant reduction in the number and intensity of skin discolorations, epidermal irregularities and porphyrin contents after 24 and 48 hours of product application.
Conclusions: The results showed a good tolerance of the study product and a significant reduction of acne lesions.
Key words: hydrogen peroxide; acne vulgaris; topical application
INTRODUCTION
Acne vulgaris (AV) is chronic inflammatory disease of sebaceous glands and hair follicles (so-called folliculosebaceous units) characterized by the presence of both non-inflammatory (i.e. microcomedones, closed comedones and open comedones) and inflammatory lesions (papules, pustules and cysts). AV is the most common skin disease in adolescents, although it is seen more and more often in adults, mostly women. It is estimated that 41% of people aged 25 to 40 suffer from acne lesions for at least 3 to 4 months per year (with a marked predominance in women – female: male ratio of 4:1) [1].
AV pathogenesis is complex, involving mostly 4 interdependent pathogenic factors: (i) increased activity of sebaceous glands with sebum overproduction; (ii) hyperkeratosis of pilosebaceous duct leading to blockage of pilosebaseous units with excess keratin and sebum, causing comedo formation; (iii) colonization of sebaceous glands ducts with anaerobic bacteria Propionibacterium acnes and increased proliferation of aerobic bacteria Staphylococcus epidermidis and lipophilic yeasts Pityrosporum ovale (which cause irritant action due to free fatty acids derived from hydrolysis of sebum triglycerides); (iv) induction of inflammatory processes in the skin. Other factors involved in the formation of acne lesions include: (i) genetic predisposition (probably polygenic or autosomal dominant with variable penetrance) – genetic factors influence density of androgen receptors in sebaceous glands, 5-α reductase activity, seborrhoea intensity and sebaceous glands size [2,3]; (ii) immunological disturbances – excessive immunological reaction (increased antibody titer and excessive inflammatory response to Propionibacterium acnes antigens, as compared to healthy population) leading to formation of inflammatory lesions, usually due to destruction of walls of the sebaceous glands; (iii) hormonal changes – subjects suffering from acne have increased 5-α reductase activity, a key enzyme involved in the transformation of testosterone into dihydrotestosterone, which strongly stimulates secretion in the sebaceous glands; (iv) environmental factors, i.e.: environmental pollution (AV occurs more often in urbanized areas), changes in climate (AV improves during spring and summer in up to 60% of patients, but usually exacerbates afterwards, i.e. in early autumn); (v) long-term stress and fatigue – acknowledged AV pathogenic factors, considered, by some authors, as main risk factors for AV in adults [4]; (vi) improper diet – so called insulinotrophic food, mostly milk and high-glycemic-acid carbohydrates (sweets and white flour food) [5,6]; (vii) taking steroids (i.e. medication or anabolic steroids used in body building and sports) – may lead to sebaceous glands hypertrophy with increased sebum secretion, increased production of skin surface lipids and increased proliferation of P. acnes [7,8]; (viii) using improper cosmetics – i.e. comedogenic (blocking pilosebaceous units) or aggressive alcoholic cosmetic preparations (which initially cause skin dryness, and thus predispose to sebum overproduction) [9].
AV occurs the most frequently in adolescents. However, it is seen more and more often in adults over 30 years of age, mostly women. It has been shown that acne lesions in women aged 30 to 40 occur more frequently in city dwellers, with higher education, performing intellectual work, and not reporting hormonal disturbances (some women report exacerbation of the symptoms in premenstrual period). This type of AV lesions usually occurs for the first time after the period of maturation in women with negative family history of AV, most frequently involves lower part of the face (mandibular and perioral areas, chin) and neck and usually presents as persistent inflammatory lesions (papules, pustules, more rarely nodules, infiltrations and cysts) resolving mostly with post-inflammatory hyperpigmentation [9].
The AV course is usually mild or moderate. However, the condition is chronic and may require treatment for several months or even years [1].
Irrespectively of age, the principles of skin care in AV remain similar. The skin care should be complex and adjusted to disease severity. In case of moderate seborrhea and mild, sparse acne lesions a reasonable skin care regimen may be sufficient. In case of persistent or severe skin lesions pharmacotherapy is usually required.
The aim of the study was to evaluate safety and anti-acne efficacy of a point-gel containing aqua, poloxamer, 2% hydrogen peroxide, 0.54% salicylic acid, 1% D-panthenol, 1% phytic acid and 0.012% niacynamide.
MATERIAL AND METHODS
The study group comprised 24 patients, 18 women and 6 men, aged 16 to 50 (mean age 24.56 ± 7.77 years), with mild to moderate AV, oily skin, having skin lesions with a tendency to form novel inflammatory lesions – papules and pustules. The patients were enrolled to the study on the basis of clinical examination by a dermatologist (during the first visit). Each patient signed an informed consent form. The study was conducted in accordance with Helsinki Declaration of 1975, as revised in 2000.
The patients had been using point-gel comprising 2% H2O2 for 7 days (composition of the product: aqua, poloxamer, hydrogen peroxide, salicylic acid, panthenol, phytic acid, polydextrose, dextrin, amylopectin, niacynamide).
The patients used the gel topically (spot application) 3 to 4 times a day during the first two days and twice a day afterwards. Twenty two patients completed the study. Two patients did not completed the study due to causes not related to the study product.
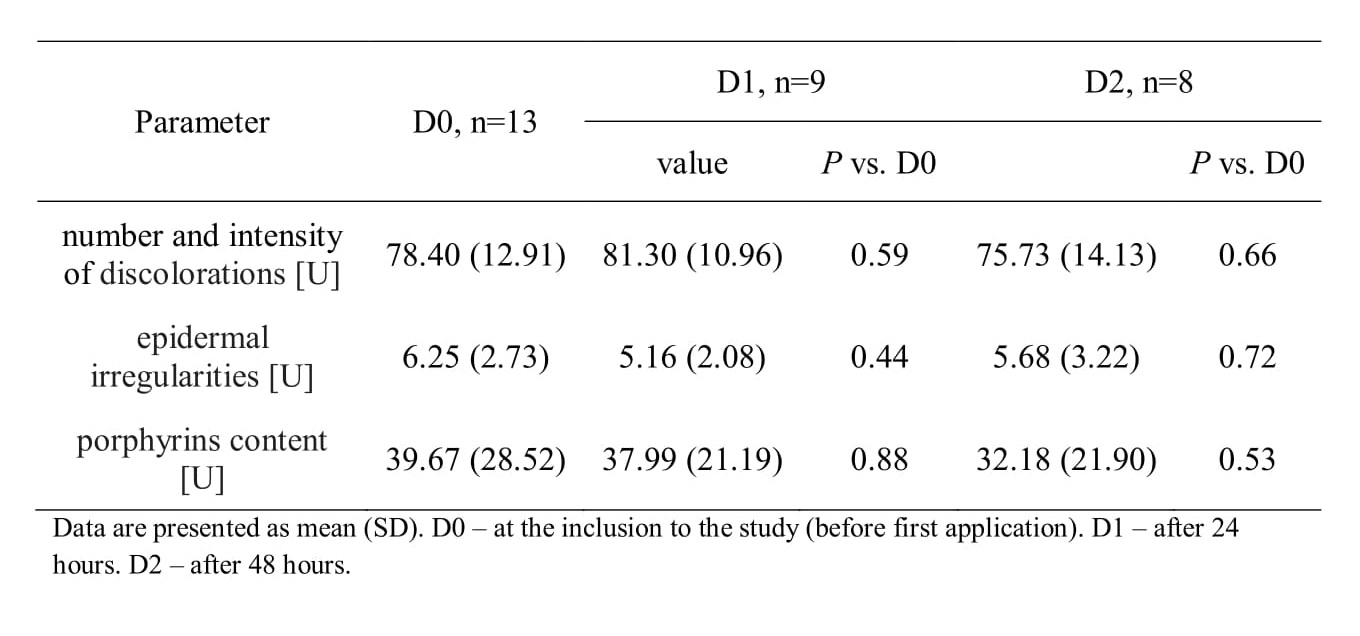
In order to assess the progress of AV treatment, additional analysis with Visia system (Canfield) was performed in selected patients before the first application and after 24 hours and 48 hours – parameters assessed included: number and intensity of skin discolorations, reduction of epidermal irregularities and porphyrins content.
The patients’ skin condition as well as the quantity and visibility of acne lesions were assessed by a dermatologist twice (during the first and last visits, i.e. Day 0 and Day 7) using of a 10-point analogue scale.
The application and skin care properties of the product were assessed during and at the end of the study with the use of a patient self-reported questionnaire.
The statistical analyses were performed using Statistica 12 package (StatSoft Inc). Results are reported as mean (SD) for continuous variables and number or % for categorical variables. The Student T test was used to compare differences between groups. P value <0.05 was considered significant.
In total, 4 reports concerning application properties of the product were recorded – mostly non-favorable appearance of the product after application on acne lesions (forming white marks). Two patients expressed dissatisfaction because of poor absorption. However, none of these 4 patients discontinued the study, and 3 of them expressed willingness to use the product in the future..
The clinical assessment by dermatologists showed a significant reduction of visibility of acne lesions (P=0.0003) and number of pustules (P=0.002). A trend toward reduction of number of comedones and papules was also observed (Table I).
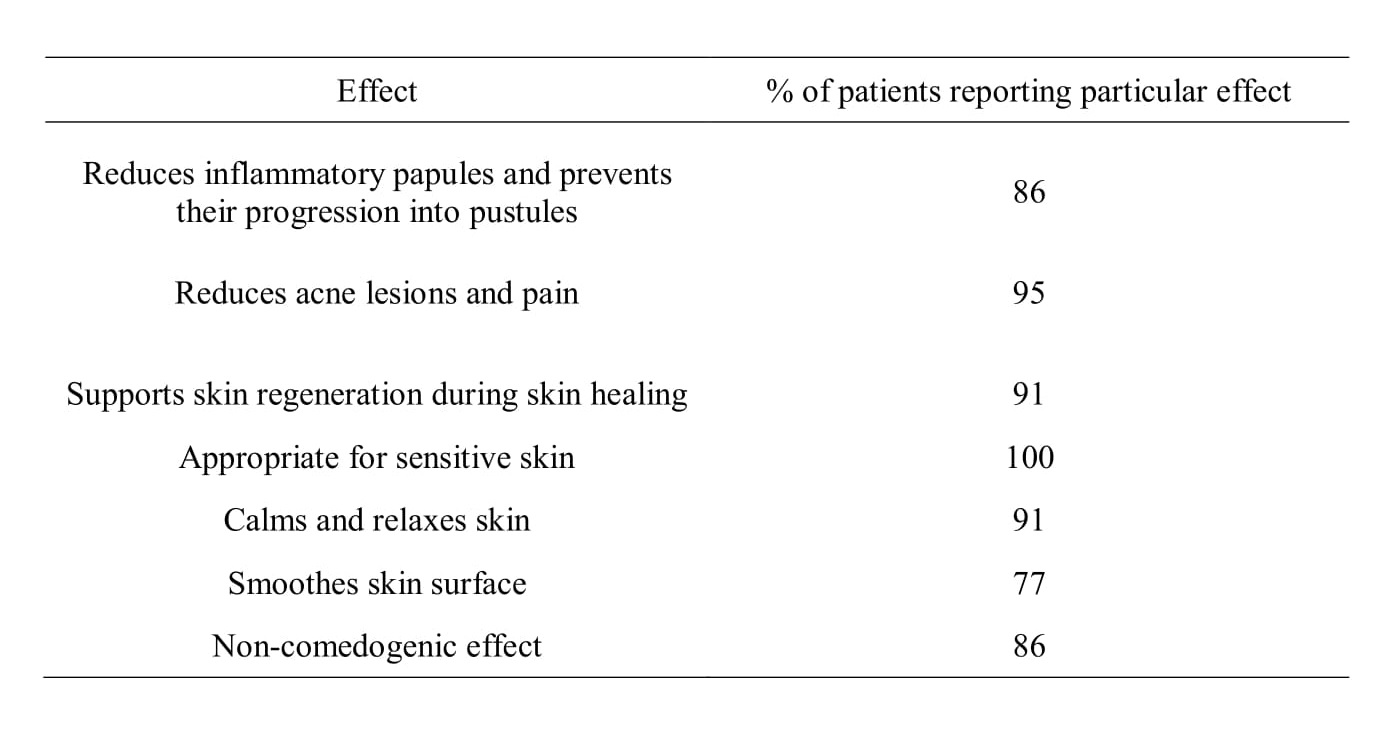
The patients reported several beneficial effects of the product at the end of the study (Table II).
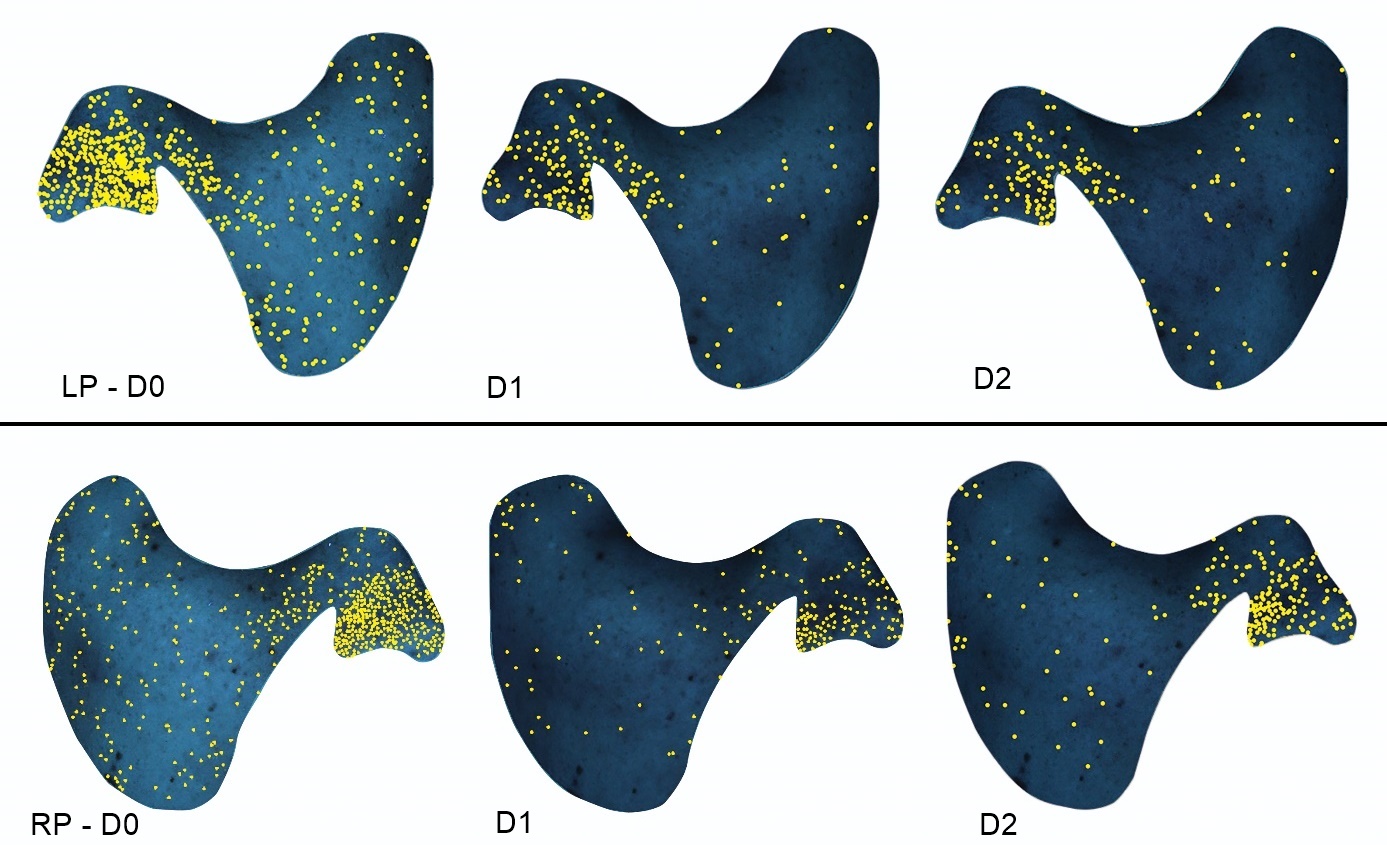
The analysis with Visia system showed a reduction of number and intensity of skin discolorations, as well as reduction of epidermal irregularities and porphyrins content after 24 and 48 hours, however the differences were insignificant (Table III, Fig. 1).
Previous studies show that reactive oxygen species – ROS (e.g. hydrogen peroxide (H2O2), peroxide (O2-), hydroxyl radical (OH) and peroxynitrite (ONOO-)) may have broad therapeutic application, i.a. improves vascularization [11,12] and wound healing [13-15], exhibit anti-cancer [16] and anti-bacterial effects [14,17]. Physiologically, ROS are involved in cell metabolism and participates in oxidation of toxic compounds. However, in high concentrations, ROS are detrimental leading to damage of the key cell structures. It is well known that ROS act as signal molecules exhibiting anti-bacterial activity in host defense reaction [18,19]. In addition, hydrogen peroxide is a biocide that has been used for disinfection and sterilization extensively. Food and Drug Administration approved hydrogen peroxide use in concentrations up to 3% (980 mM) [20].
The anti-bacterial activity of H2O2 was the basis for developing a point gel which acts locally on bacteria promoting AV. Hydrogen peroxide is disintegrated to water and oxygen by catalase – an enzyme found in all aerobic organisms, as well as in facultative anaerobes. However, obligate anaerobes does not produce at all or produce catalase in very small quantities. This fact explains the ability of H2O2 to eliminate anaerobic pathogens, including those responsible for AV (P. acnes) [21,22].
Our study showed, that 2% hydrogen peroxide was effective in mild to moderate acne. Products containing 3% hydrogen peroxide cause local skin discoloration and are used for skin disinfection. A study by Fabbrocini and Panariello showed efficacy of a locally applied gel containing 3% hydrogen peroxide, 1.5% salicylic acid and 4% D-panthenol in the management of mild to moderate acne. The product was applied on the whole skin surface for 60 days and was shown to have high efficacy as compared to previous version of product containing higher H2O2 and lower salicylic acid concentrations (4 and 0.5%, respectively) [23].
Ricci et al. found that products containing hydrogen peroxide, salicylic acid and D-panthenol may be used during sun exposure [24]. A study by Capizzi et al. showed better skin tolerability and efficacy of combination therapy with hydrogen peroxide stabilized cream and adapalene gel as compared to benzoyl peroxide cream and adapalene gel in mild to moderate acne [25]. A better skin tolerability of hydrogen peroxide gel in comparison to 4% benzoyl peroxide was also found in previous studies [26]. On the other hand, a study by Veraldi et al. showed a similar effect of hydrogen peroxide and benzoyl peroxide on inflammatory and non-inflammatory skin lesions in mild to moderate AV [27].
The 2% H2O2 gel assessed in the current study did not cause skin discoloration, was well tolerated and had a pleasant (according to patients’ questionnaires) consistence. The product was enriched in compounds potentiating anti-acne effect of hydrogen peroxide, i.e. salicylic acid, D-panthenol, phytic acid and niacynamide.
The study product contained salicylic acid in a concentration of 0.54% (similarly to the study by Fabbrocini and Panariello [23]). Salicylic acid exhibits anti-bacterial, anti-seborrhoeic and lightening effect. It has a beneficial effect in the treatment of AV, both in case of comedonal acne with sparse inflammatory lesions, and in the management of chronic acne in adults [28].
D-panthenol is a non-toxic, gentle for skin and non-comedogenic compound. It improves skin humidity and thus, reduces pruritus and makes skin less prone to inflammation. The soothing effect of panthenol leads to reduction of redness and irritations. It also exhibits regenerative effect, thus accelerating healing of small wounds, scratches and abrasions. It explains why D-panthenol is one of basic compounds used in dermocosmetics for sensitive and irritated skin, and skin with dermatologic problems.
Phytic acid naturally occurs in most grains (e.g. cereal grains and brans, corn and leguminous crops). It is a chelating agent suppressing iron and copper penetration into cells, and thus decreasing melatonin production. It explains its use in whitening creams [29-31] and peelings dedicated for acne skin [32]. Besides skin-lightening and anti-hyperpigmentation effects, phytic acid also exhibits anti-inflammatory and anti-oxidant activity [33]. Furthermore, it does not sensitizes skin to UV radiation, and thus, may be used during summer. Products containing phytic acid are characterized by good stability and good hydrating properties [31].
Niacynamide (used in the study product in low concentration) has a delicate lightening effect, improves skin texture and exhibits anti-inflammatory activity [34]. Hence, it constitutes a beneficial addition to cosmetic therapy in AV patients.
AV is a potentially chronic disease that needs complex management. One of the key issues is proper skin care with the use of gentle and non-comedogenic, but, at the same time, effective dermocosmetics. In case of non-inflammatory lesions, the use of proper anti-acne product may decrease the risk of inflammatory lesions (i.e. papules and pustules). However, in severe cases dermatologic treatment is necessary to prevent formation of persistent lesions, e.g. scars and discolorations [35].
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
REFERENCES
2. Xu SX, Wang HL, Fan X, Sun LD, Yang S, Wang PG. The familial risk of acne vulgaris in Chinese Hans – a case-control study. J Eur Acad Dermatol Venereol. 2007;21:602-5.
3. Lichtenberger R, Simpson MA, Smith C, Navarini AA. Genetic architecture of acne vulgaris. J Eur Acad Dermatol Venereol. 2017;31:1978-90.
4. Chiu A, Chon SY, Kimball AB. The response of skin disease to stress: changes in the severity of acne vulgaris as affected by examination stress. Arch Dermatol. 2003;139:897-900.
5. Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;15:371-88.
6. Melnik BC. Acne vulgaris. Role of diet. Hautarzt. 2010;61:115-25.
7. Szepietowski J, Kapińska-Mrowiecka M, Kaszuba A, Langer A, Placek W, Wolska H. Trądzik zwyczajny: patogeneza i leczenie. Konsensus Polskiego Towarzystwa Dermatologicznego. Przeg Dermatol. 2012;99:649-73.
8. Melnik BC, Jansen T, Grabbe S. Abuse of anabolic-androgenic steroids and bodybuilding acne: an underestimated health problem. J Dtsch Dermatol Ges. 2007;5:110-7.
9. Chlebus E, Chlebus M. Factors affecting the course and severity of adult acne. Observational cohort study. J Dermatol Treat. 2017;28:737-44.
10. Kisiel K, Dębowska R, Pasikowska M, Vincent C, Cieścińska C, Chełstowska-Paździorko J. Ocena skuteczności i bezpieczeństwa stosowania dermokosmetyków do mycia ciała i włosów u dzieci z atopowym zapaleniem skóry. Dermatol Dziec. 2016;6:42-5.
11. Breton-Romero R, Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529-34.
12. Lee SH, Boire TC, Lee JB, Lee JB, Gupta MK, Zachman AL, et al. ROS Cleavable proline oligomer crosslinking of polycaprolactone for pro-angiogenic host response. J Mater Chem B. 2014;2:7109-13.
13. Loo AE, Ho R, Halliwell B. Mechanism of hydrogen peroxide-induced keratinocyte migration in a scratch-wound model. Free Radical Biol Med. 2011;51:884-92.
14. Lee Y, Choi KH. In situ forming and H2O2-releasing hydrogels for treatment of drug-resistant bacterial infections. ACS Appl Mater Interfaces. 2017;9:16890-9.
15. Strong AL, Nauta AC, Kuang AA. Local wound care for primary cleft lip repair: Treatment and outcomes with use of topical hydrogen peroxide. Wounds. 2015;27:319-26.
16. Raj L, Ide T, Gurkar AU. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nat Lett. 2011;475:231-4.
17. Michl TD, Coad BR, Doran M, Osiecki M, Kafshgari MH, Voelcker NH. Nitric oxide releasing plasma polymer coating with bacteriostatic properties and no cytotoxic side effects. Chem Commun. 2015;51:7058-60.
18. Rhee SG. Cell Signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882-3.
19. Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev. 2013;37:955-89.
20. Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67:1589-96.
21. Scibor D, Czeczot H. Katalaza – budowa, właściwości, funkcje, Postepy Hig Med Dosw. 2006;60:170-80.
22. Hentges DJ: Anaerobes: general characteristics, In: Medical Microbiology. Samuel Baron (eds). 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston. 1996:17.
23. Fabbrocini G, Panariello L. Efficacy and tolerability of a topical gel containing 3% hydrogen peroxide, 1,5% salicylic acid and 4% D-panthenol in the treatment of mild-moderate acne. G Ital Dermatol Venereol. 2016;151:287-91.
24. Ricci F, Masini F, Fossati B, Frascione P, Waure CDE, Capizzi R, et al. Combination therapy with hydrogen peroxide (4%), salicylic acid (0,5%) and D-panhenol (4%): efficacy and skyn tolerability in common acne vulgaris during sun exposure period. Eur Rev Med Pharmacol Sci. 2016;19:232-6.
25. Capizzi R, Landi F, Milani M, Amerio P. Skin tolerability and efficacy of combination therapy with hydrogen peroxide stabilized cream and adapalene gel in comparison with benzoyl peroxide cream and adapalene gen in common acne. A randomized, investigator-masked, controlled trial. Br J Dermatol. 2004;151:481-4.
26. Milani M, Bigardi A, Zavattarelli M. Efficacy and safety of stabilised hydrogen peroxide cream (Crystacide) in mild-to-moderate acne vulgaris: a randomised, controlled trail versus benzoyl peroxide gel. Curr Med Res Opin. 2003;19:135-8.
27. Veraldi S, Micali G, Berardesca E, Dall’Oglio F, Sinagra JL, Guanziroli E. Results of a multicenter, randomized, controlled trial of a hydrogen peroxide-based kit versus a benzoyl peroxide-based kit in mild-to-moderate acne. J Clin Aesthet Dermatol. 2016;9:50-4.
28. Chlebus E, Serafin M. Peelingi chemiczne wczoraj i dziś (2). Nowe podejście do mechanizmów oddziaływania substancji złuszczających na skórę. Dermatol Estet. 2015;17:171-9.
30. Levy JL, Pons F, Agopian L, Besson R. A double-blind controlled study of a nonhydroquinone bleaching cream in the treatment of melasma. J Cosmet Dermatol. 2005;4:272-6.
31. Okazaki Y, Sekita A, Katayama T. Intake of phytic acid and myo-inositol lowers hepatic lipogenic gene expression and modulates gut microbiota in rats fed a high-sucrose diet. Biomed Rep. 2018;8:466-74.
32. Al-Mokadem S, Al-Aasser O, Nassar A, Al-Sharkawy AE. Easy phytic peel as a therapeutic agent in acne vulgaris and melasma. Egyp J Dermatol Venereol. 2013;33:6–11.
33. Manosroi A, Chutoprapat R, Sato Y, Miyamoto K, Hsueh K, Abe M, et al. Antioxidant activities and skin hydration effects of rice bran bioactive compounds entrapped in niosomes. J Nanoscien Nanotechnol. 2011;11:2269–77.
34. Bissett DL, Oblong JE, Berge CA. Niacinamide: A B vitamin that improves aging facial skin appearance. Dermatol Surg. 2005;31:860-5.
35. Harald PM, Gollinck MD. Managing acne in the middle east: consensus recommendations. JEADV. 2017;31:suppl.7:1-35.
Notes
Source of Support: This study was funded by Laboratorium Kosmetyczne Dr Irena Eris Sp. z o.o. (Warsaw, Poland). Laboratorium Kosmetyczne Dr Irena Eris Sp. z o.o. sponsored the study, participated in design of the study as well as in the collection, analysis, interpretations of the data and preparing final version of the manuscript. However, the decision to submit and publish this manuscript was contingent on the approval of the lead author and all co-authors,
Conflict of Interest: None declared.
Comments are closed.