Dermoscopic features of vulvar sclerosus and atrophic lichen: About 52 cases
Ryme Dassouli , Hanane BayBay, Nora Kalmi, Kenza Tahiri Joutei, Zakia Douhi, Sara Elloudi, Fatima Zahra Mernissi
, Hanane BayBay, Nora Kalmi, Kenza Tahiri Joutei, Zakia Douhi, Sara Elloudi, Fatima Zahra Mernissi
Department of Dermatology, University Hospital Hassan II, Fès, Morocco
Corresponding author: Ryme Dassouli, MD
Submission: 02.09.2021; Acceptance: 19.02.2022
DOI: 10.7241/ourd.2022e.9
Cite this article: Dassouli R, BayBay H, Kalmi N, Joutei KT, Douhi Z, Elloudi S, Mernissi FZ. Dermoscopic features of vulvar sclerosus and atrophic lichen: About 52 cases. Our Dermatol Online. 2022;13(e):e9.
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Vulvar lichen sclerosus is a chronic inflammatory disease of the vulva. Its clinical aspect can be confused with other inflammatory or neoplastic vulvar diseases. However, thanks to dermoscopy, which is a non-invasive means of exploration, certain signs are very helpful for the diagnosis. Nowadays, specific dromoscopic models of LSV are being developed.
Objective: The objective of our study was to detect the specific dermoscopic characteristics of LSV.
Method: We carry out a retrospective study including 52 patients who consulted the Hassan II University Hospital of Fez over a period from August 2019 to April 2021. A photographic dermoscopic exploration was performed on the LSV lesions of our patients and archived for analysis.
Results: Decreased total vascularity, comedo type plugs, and the appearance of burst ice represented new specific dermosocpic signs. Our study denies the presence of specific demoscopic signs of LSV to predict the coexistence of lichen on another anatomical location.
Conclusion: Dermoscopy has been proven to be effective in the diagnosis of LSV. However, more studies should be done to better highlight the role of dermoscopy in the therapeutic follow-up of LSV.
Key words: Vulvar lichen sclerosus, Dermoscope, Vascular pattern, Non-vascular pattern
INTRODUCTION
Vulvar lichen sclerosus (LSV) is a chronic inflammatory disease affecting the anogenital regions with a huge impact on the quality of life. The risk of progression to squamous cell carcinoma is not negligible [1,2].
LSV may mimic other inflammatory or tumorous vulvar diseases. The histopathological examination helps to decide on the diagnosis. Dermoscopy, a non-invasive tool, has proven its usefulness in the diagnosis, management and prognosis of LSV [3]. Nowadays, current studies aim to provide specific dermoscopic models of LSV making it possible to distinguish it from other inflammatory genital diseases before performing histopathological confirmation, and also for a better diagnostic orientation, an early and adapted management and a better prognosis [4,5]. However, there are no studies demonstrating specific dermoscopic signs of LSA associated with lichen on other sites. The present study was designed to describe the dermoscopic features of a large series of 52 cases of LSV and to analyze the dermoscopic signs appearing on an exclusive LSV and the cases of LSV associated with another location of lichen.
MATERIALS AND METHODS
Objective
The objective of our study was to describe the dermoscopic characteristics of LSV involving the vulvar unit associated or not with another lichen localization on a sample of patients collected at the consultation of the Dermatology Department of the Hassan II University Hospital of Fez, over a period from November 2020 to July 2021. We compared the results of our study to the literature data in order to differentiate the specific signs of LSV from other inflammatory or neoplastic diseases of the vulva. We also compared the dermoscopic results of exclusive LSV with LSV combined with another lichen in our series, and performed an analytical study with the help of the epidemiology department of the Hassan II University Hospital of Fes, using the Khi-2 test and Fisher’s exact test to find the significant signs of LSV combined with another location.
Patients and data collection
We retrospectively analyzed a total of 481 dermoscopic photos of 52 patients followed in consultation with the dermatology department of CHU Hassan II in Fez. All patients had a histologically confirmed diagnosis of LSV. The biopsy was performed with a dermoscope orientation from the most important site. All the patients were in the active phase of the disease. Clinical lesions examined by dermoscopy were indurated hypopigmented patches or papules with or without pink and/or brown macules. The presence of LS in other skin or mucosal sites was not an exclusion criterion.
Dermoscopic evaluation
The dermoscopic examination was performed during the routine dermatological consultation. The analyzed pictures were taken with a digital dermoscopy system (Dermatoscope DermliteDl4) in polarized mode. Minimal pressure was applied to preserve vessel morphology, and immersion with a clear colorless gel was used to ensure better visualization. The instrument was packaged without disposable food packaging to avoid microbiological contamination. Dermoscopic image capture was performed by the same dermatologist to avoid diversification during the procedure. As several different images were captured in each patient, the evaluation of the images consisted in searching in the set of images taken the characteristic dermoscopic criteria of the ASL, and to realize a descriptive study calculating the percentages and the frequencies of the dermoscopic signs on the one hand, and an analytical study analyzing the specificity of the dermoscopic signs of the ASL associated with another anatomical localization. On the other hand, the evaluation of the images consisted in searching in the set of images taken the characteristic dermoscopic criteria of LSV and in calculating their frequencies, and on the other hand in analyzing the specificity of the dermoscopic signs of LSV in case of association with another clinical localization, and this with the help of the service of epidemiology based on the test of chi-2 and the Test exact of Fisher.
Selection of variables
The selection of dermoscopic variables included in the evaluation process was based on the literature and personal expertise [6,7]. The variables included in the dermoscopic assessment were: vascular pattern (dotted, linear, sperm-like, polymorphic vessels); non-vascular structures: white areas without structures; structures like ice slivers, milky red areas; peppery appearance; brown areas without structures; brownish linear crosslinks; purpuric spots/globules/plaques; comedo-like openings.
RESULTS
The average age of the patients included in our study was 50.4 years, ranging from a minimum age of 7 years to a maximum age of 86 years. The clinical presentation of LSV was dominated by whitish sclerotic plaques, hooding of the clitoris with effacement of the labia minora, with the presence of homogeneous pigment spots on the plaques (Fig. 1).
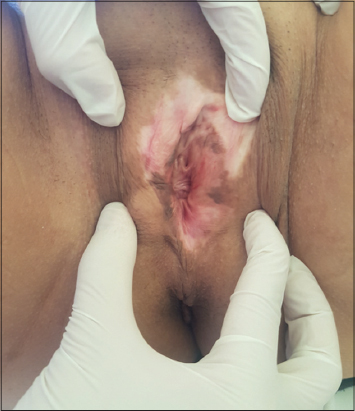 |
Figure 1: Whitish sclerotic plaques, hooding of the clitoris with effacement of the labia minora, with the presence of homogeneous pigment spots on the plaques. |
The results of our study describe a vascular and a nonvascular pattern. The vascular pattern is dominated by the decrease in total vessel density with a percentage of 70.37% (Fig. 2a). Polymorphic vascular layers in 51.85% made essentially of a mixture of linear vessels, spotted or globular vessels and telangiectasias. Linear vessels were observed in 48.14% of cases and appeared as red telangiectasias of different calibers and sizes (Fig. 2b). Mottled vessels were observed in 33.33% of cases. Sperm-like vessels were not observed in any patient. The architectural organization of the vascular vessels was mainly dominated by irregular arrangements with a percentage of 48.14%. Vascular distribution at the periphery of the plaques was not observed in any case. Well-defined red to purpuric spots, cells, or dots, corresponding to blood spots (Fig. 2c), were observed in 20 patients (36.46% of cases).
Regarding the non-vascular pattern, the vast majority of lesions had a whitish background, 72.22% of cases, with irregular whitish areas without structures in 62.96% of cases, and milky red areas (Figs. 2d and 2e) in 55.55% of cases. Structure-free areas of different hues frequently coexist in the same patient according to the results of series reported in the literature.
Ice chip-like structures were observed in the whitish areas in 37.03% of patients. These bright, clearly delineated white areas appeared in a linear or triangular configuration (Figs. 2f and 2g).
Yellowish comedo-like openings (Fig. 2d), especially on the skin side of the vulvar lesions, were present in 42.59% of cases. Brown areas without structures or brownish linear reticulations were observed in 35.18% of cases and a peppery appearance in 48.14% of cases.
The analytical study measuring by cross-tabulations the association of each dermoscopic sign found with the coexistence of an extravulvar lichen was negative with p values higher than 0.05 for each variable.
There was also no significance between the age of the patients and the possibility of coexistence of LSV with another anatomical location (p=0,715).
DISCUSSION
Vulvar lichen sclerosus is a significant inflammatory disease that is common especially in women in their 50s and beyond. If left untreated, it can cause significant and permanent deformation of the vulvar structure with a 2-6% lifetime risk of scaly malignant neoplasia of the vulva. These complications can be avoided with early recognition and timely intervention [7].
In recent years, dermoscopic examination has proven to be a useful support to aid in the recognition/non-invasive differential diagnosis of many vulvar inflammatory diseases including LSV [3–5]. The dermoscopic features of LSV have been described in a few reports and case series [1].
Nevertheless, histopathology is an essential element to confirm the diagnosis. An important biopsy fragment guided by clinical data and dermoscopic allows diagnosis and detection of histopathological changes and dysplastic or degenerative changes.
Our observations indicate that the observed vascular and non-vascular dermoscopic patterns are quite characteristic of LSV. Our observations indicate that LSV presents a rather characteristic pattern. It combines a vascular pattern made mainly of linear or dotted vessels sparse irregularly on a pale background and areas without whitish or milky pink structures with variable and degraded shades, homogeneous areas or brownish reticulations, and a peppery appearance.
The results of our study were roughly similar to studies in the literature on LSV dermoscopy [2,6,8]. A whitish background (Figs. 1 and 2a) as well as areas without uneven structure, varying in color from white to yellowish-white to milky pink (Figs. 2b and 2c), represent the predominant dermoscopic feature of LSV. These dermoscopic signs can also be observed in the absence of paleness at clinical evaluation. They correspond to sclerosis and hyalinization, which are the main pathological changes in lichen sclerosus (LS) [6]. A marked decrease in vascular concentration in the sclerotic plaques of LSV compared to unaffected areas and a very frequent dermoscopic feature in our series. Convincing the vascular pattern, Vessels are polymorphic in shape, made up of a combination of linear vessels and dot vessels with arborescent telangiectasias. The arrangement was mostly irregular or in some cases grouped in the center of the plates. The correlation between dermoscopic vascular pattern and disease duration is controversial. Indeed, according to Borghi and his colleagues, it is likely that the dotted vessels occur mainly in the early stages of the disease, and correspond to the dilated blood vessels under the basement membrane. On the other hand, in later stages, cutaneous fibrosis causes vascular changes and causes their disappearance [6]. While Larre Borges and his colleagues found no association. The correlation between dermoscopic vascular pattern and disease duration is controversial. Indeed, according to Borghi and his colleagues, it is likely that the dotted vessels occur mainly in the early stages of the disease, and correspond to the dilated blood vessels under the basement membrane. On the other hand, in later stages, cutaneous fibrosis causes vascular changes and causes their disappearance [6]. While Larre Borges and his colleagues found no association. The correlation between dermoscopic vascular pattern and disease duration is controversial. Indeed, according to Borghi and his colleagues, it is likely that the dotted vessels occur mainly in the early stages of the disease, and correspond to the dilated blood vessels under the basement membrane. On the other hand, in later stages, cutaneous fibrosis causes vascular changes and causes their disappearance [6]. While Larre Borges and his colleagues found no association. cutaneous fibrosis causes vascular changes and causes their disappearance. While Larre Borges and his colleagues found no association. cutaneous fibrosis causes vascular changes and causes their disappearance [6]. While Larre Borges and his colleagues found no association.
Particular structures like ice slivers, linear or triangular or lanceolate in shape, have been observed within VLS lesions. It can be said that these dermoscopic features correspond to hyperkeratosis of the adnexal structures. Ice shard-shaped structures should not be confused with pupa structures [9–12], which may occasionally be observed in extragenital LS under polarized dermoscopy.
A valuable dermoscopic structure that stood out in our series, it has yellow openings resembling comedones-Like were found in 42.59% of patients, mainly on the skin side of the SLV lesions. These structures are described in studies describing the dermoscopic aspects of extragenital LS. Histologically, they correspond to dilated infundibula filled with keratin [7–9,12].
Gray-blue dots arranged in a typical peppery pattern have been frequently observed in our patients, corresponding to melanophages displaced in the upper dermis and at the level of the perifollicular sites which is a consequence of the inflammatory process, which explains why it is also observed in other chronic genital inflammatory diseases. Thus, pepper cannot be considered as a diagnostic clue for VLS [2,5].
Red blood cells or spotswith well-circumscribed purpuric are common dermoscopic findings in LSV, which correspond to blood spots [2,10,11]. Their disappearance is a good marker of therapeutic response, as some authors have announced.
To our knowledge, no specific signs of vulvar lichen scleratrophic have ever been noted in the literature that intersect with other cutaneous or mucosal locations of lichen. We did a cross study between dermoscopic signs of exclusive vulvar lichen sclerosus and patients with both vulvar and cutaneous involvement of lichen, and in accordance with the literature, there were no specific dermoscopic signs of lichen scleratrophy in case of overlap with other localization of lichen.
In addition, according to our study, there are no dermoscopic vulvar signs that can predict the presence of lichen on another topography of the body, cutaneous or mucosal. however, the sample studied of cross-localization of lichen was not numerous enough to be able to draw definitive rules. Further studies are necessary to confirm or refute our finding.
The dermoscopic evaluation not only makes it possible to evaluate the therapeutic response of the disease and to detect the dysplastic evolution, but also makes it possible to make the differential diagnosis with other inflammatory pathologies in the vulva [13].
CONCLUSION
recognition of specific dermoscopic models of LSV can help diagnose the disease at an early stage and improve differential diagnosis with other inflammatory genital diseases, especially when there is clinical doubt.
Through our descriptive and analytical study, we highlight the characteristic dermoscopic signs of LSV, such as the decrease of total vascularization, comedon-like plugs, the appearance of exploded ice. Our study denies the presence of specific demoscopic signs of LSV predicting the coexistence of a lichen on another anatomical location. We also did not find any significance between the young age and the presence of another location of the lichen in association with the LSV. Since our Since our crossover sample is small, further studies are needed to confirm the results of our study.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Liu J, Hao J, Liu Y, Lallas A. Dermoscopic features of lichen sclerosus in Asian patients:a prospective study. J Eur Acad Dermatol Venereol. 2020;34:720-1.
2. Borghi A, Corazza M, Minghetti S, Bianchini E, Virgili A. Dermoscopic features of lichen sclerotic vulva in a prospective cohort of patients:new observations. Dermatology. 2016;232:71–7.
3. Errichetti E, Stinco G. The practical useful- ness of dermoscopy in general dermatology. G Ital Dermatol Venereol. 2015;150:533–46.
4. Russo T, Piccolo V, Lallas A, Argenziano G. Recent advances in dermoscopy. F1000Res. 2016;5:184.
5. Borghi A, Virgili A, Corazza M. Dermoscopy of inflammatory genital diseases:practical insights. Dermatol Clin. 2018;36:451-61.
6. Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, Moscarella E, Gurgitano S, Capurro M, et al. Clinical, dermoscopic and histopathological features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2013;27:1433–9.
7. Lee A, Fischer G. Diagnosis and treatment of vulvar lichen sclerosus:an update for dermatologists. Am J Clin Dermatol. 2018;19:695-706.
8. Horcajada-Reales C, Campos-DomínguezM, Conde-Montero E, Parra-Blanco V, Suárez- Fernández R. Comedo-type openings in dermoscopy:an essential diagnostic clue for lichen sclerosus, even in children. J Am Acad Dermatol. 2015;72:4-5.
9. Lacarrubba F, Pellacani G, VerzìAE, Pippo M, Micali G. extragenital lichen sclerosus:clinical, dermoscopic, confocal and histological correlations. J Am Acad Dermatol. 2015;72:50-2.
10. Oakley A. Dermatoscopic features of vulvar lesions in 97 women. Australas J Dermatol. 2016;57:48-53.
11. Lacarrubba F, Dinotta F, Nasca MR, Fabbrocini G, Micali G. Localized vascular lesions of the glans in patients with lichen sclerosus diagnosed by dermatoscopy. G Ital Dermatol Venereol. 2012;147:510-1.
12. Marghoob AA, Cowell L, Kopf AW, Scope A. Observation of chrysalis structures by polarized dermoscopy. Arch Dermatol. 2009;145:618.
13. Armor M, Virgili A, Minghetti S, Toni G, Borghi A. Dermoscopy in balanitis plasma cells:its usefulness in diagnosis and monitoring. J Eur Acad Dermatol Venereol. 2016;30:182-4.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-4330-4429 http://orcid.org/0000-0003-4330-4429 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0002-5942-441X http://orcid.org/0000-0002-5942-441X |




Comments are closed.