Adverse effects of propranolol in a fulminant ulcerated infantile hemangioma
Aida Oulehri , Hanane Baybay, Sara Elloudi, Zakia Douhi, Fatima Zahra Mernissi
, Hanane Baybay, Sara Elloudi, Zakia Douhi, Fatima Zahra Mernissi
Department of dermatology, University Hospital Hassan II, Fez, Morocco
Corresponding author: Aida Oulehri, MD
How to cite this article: Oulehri A, Baybay H, Elloudi S, Douhi Z, Mernissi FZ. Adverse effects of propranolol in a fulminant ulcerated infantile hemangioma. Our Dermatol Online. 2022;13(e):e19.
Submission: 27.12.2020; Acceptance: 06.03.2021
DOI: 10.7241/ourd.2022e.19
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Infantile hemangiomas (IHs) are the most common tumors of infancy, with a global prevalence estimated at 1% to 10% of infants. Most hemangiomas are small and regress spontaneously without a need for intervention. However, 5% to 10 % of hemangiomas may cause serious complications and require treatment. Herein, we report the case of a two-month-old female infant with a left hemifacial hemangioma, which became severely ulcerated after the introduction of propranolol. We also report a good improvement afterwards due to combined therapy. An ulcerated infantile hemangioma is the most common complication seen in an IH, which may present a therapeutic challenge especially in the case of concurrent hemorrhage or infection. Although numerous studies support its efficacy and reduced side effects. propranolol may worsen the ulceration of an IH, possibly reflecting a reduced blood flow, causing peripheral ischemia. In some cases of IH with complex ulceration, monotherapy with propranolol may be insufficient.
Key words: Fulminant ulceration; Infantile hemangioma; Propranolol
INTRODUCTION
Infantile hemangiomas (IHs) are the most common type of vascular tumors derived from vascular endothelial cells [1], generally occurring around one week after birth, with a male-to-female ratio of approx. 1:3 [2]. Numerous mechanisms have been proposed to explain the pathogenesis of IH, but the exact etiology is yet to be fully described. Several hypotheses are favored, including 1) endothelial stem or progenitor cells migrating to locations and proliferating abnormally; 2) the overexpression of the vascular endothelial growth factor (VEGF) receptor resulting in vascular cell proliferation; 3) lesions arising from ectopic placental tissue and exhibiting similar protein expression and life cycle as the placental tissue; and 4) localized hypoxia as a factor initiating the development or proliferation of an IH [3]. Prematurity, low birth weight, the female sex, and the white race are common risk factors associated with the development of IH [4]. The natural course of IH has been well described and is characterized by a rapid proliferative phase, followed by a quiescent plateau phase, and finally by a multiyear involutional phase [5]. Although most hemangiomas are small and regress spontaneously without a need for intervention, approx. 15% of IHs result in complications, such as the obstruction of the airway and vision, bleeding, ulceration, and disfigurement accompanied by underlying abnormalities, and so they require therapeutic intervention [6].
CASE REPORT
A two-month-old female infant born at term to a 38-year-old mother and a 34-year-old father, with no notable pathological antecedents. The examination at birth was normal. On day seven of the infant’s life, the mother noticed telangiectasic lesions on the left hemifacial area, then the progressive installation of a centrifugal violet erythematous patch on the left lateral side of the scalp, the auricle of the ear, the parotid region, and the neck. On week three of life, three small ulcerations in the temporal region appeared. The diagnosis of a segmental infantile hemangioma was reached. The infant was treated with propranolol at a dose of 3 mg/kg/day.
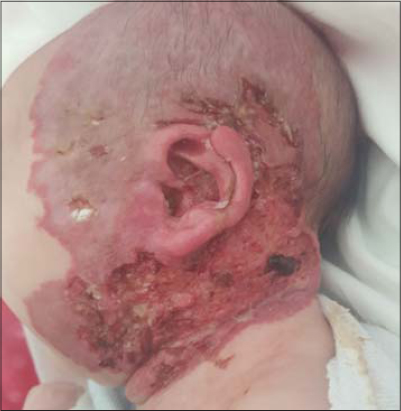
Fifteen days later, the mother brought the infant again with the fulgurant extension of the ulceration, which had rapidly expanded, both superficially and deeply (Fig. 1), affecting the entire left lateral aspect of the neck with extension toward the chin and the retroauricular region, complicated by the perforation of the auricle (Fig. 2).
Faced with such dramatic evolution, our decision was to maintain beta blockers at the same dose, add an oral corticosteroid in the form of betamethasone at a dose of 3 mg/kg/day, and associate two sessions a week of a pulsed dye laser after the use of eosin as a photosensitizer in addition to ulcer care with hydrocolloid dressings every other day.
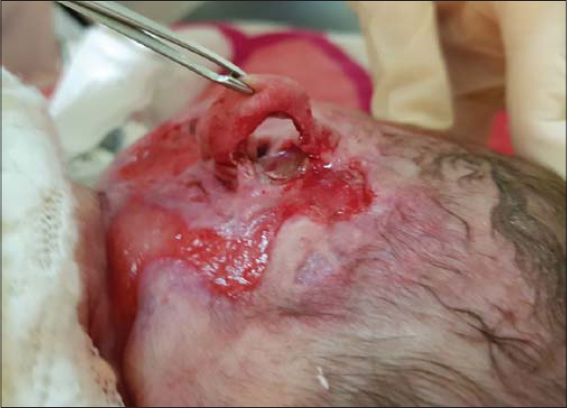
Now the evolution was favorable: the progression of ulceration stopped and the HI started to gradually regress. After one month of treatment, the evolution was favorable, with complete healing of the ulcer and partial regression of the hemangioma (Fig. 3). Basing on an abdominal and transthoracic ultrasound, we also performed an extended assessment in the face of a suspected PHACES syndrome, which returned to normal. Unfortunately, cerebral MRI had not yet been performed.
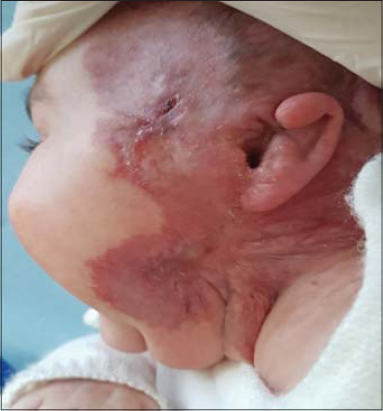 |
Figure 3: Favorable evolution with complete healing of the ulcer and partial regression of the hemangioma after one month of treatment. |
Now, the little girl is doing very well. The hemangioma is regressive. She is currently two-years-old (Fig. 4). A treatment for the scar is in progress.
 |
Figure 4: Setback at the age of two years. |
DISCUSSION
Ulceration is the most common complication seen in IH, with its prevalence varying from 15% to 25% according to various authors [4,7,8]. Ulceration is most commonly observed between the fourth and eighth months of life [4], but sometimes as early as in the neonatal period [8]. An IH may be described as superficial, deep, or mixed (with both superficial and deep components). Superficial and mixed hemangiomas are more likely to ulcerate, whereas deep hemangiomas rarely do [7]. Size and location are also determinants of ulceration, with large and segmental hemangiomas being substantially more likely to ulcerate. Predilection areas for ulceration are the lips, the head and neck area, and the intertriginous regions. Constant exposure to moisture and maceration seem to promote ulceration [9]. An IH tends to ulcerate during the proliferative phase. Furthermore, some clinical forms of IH—such as telangiectatic IH of the head and genitals, rapidly-growing neonatal IH with a shiny-red appearance, and segmental facial IH—are more often complicated by ulceration [7]. Although the pathogenesis of such ulceration is unknown, three factors are thought to play a role: 1) sites of trauma (maceration and friction), 2) local factors such as bacterial infection or colonization, and 3) tissue hypoxia such that ulceration occurs when the hemangioma “outgrows its blood supply” [10].
Usually, the first clinical signs include blackish spots on the surfaces of red zones [8]. Moreover, white discoloration of an IH or its margins appears to be highly sensitive and relatively specific for predicting ulceration in an IH [11,12]. Such ulceration may be very painful and complicated by secondary infection and bleeding, which may lead to severe anemia and the need for transfusion. Finally, disfiguring scarring usually follows wounding [8].
Ulceration is always an indication for intervention and, currently, there are numerous options for its treatment, which may be broadly categorized into wound care or conservative management, medical therapy, laser therapy, and surgery. The treatment of such ulceration falls into three categories: halting proliferation with therapy, altering the local environment, and pain management.
Due to its impressive efficacy and safety profile, propranolol has become the first-line therapy for IH worldwide in a remarkably short time, with a recommended dose of 2–3 mg/kg/day [3,6,13].
The exact mechanism of action of propranolol on an IH is still not completely understood. Recent studies have offered evidence for a variety of mechanisms, including pericyte mediated vasoconstriction, the inhibition of vasculogenesis, catecholamine-induced angiogenesis, and the downregulation of the renin–angiotensin–aldosterone axis [13].
An ulcerated IH requires treatment with propranolol if topical treatment has not been effective or is not appropriate, as in large IHs that cannot be treated with topical timolol maleate. An ulcerated IH beyond the growth phase does not require treatment with propranolol. Propranolol may very rarely worsen the ulceration of an IH, possibly reflecting a reduced blood flow, causing peripheral ischemia. In such cases, a reduction in the dose of propranolol may be helpful [13].
The major adverse events occurring in patients with IH treated with propranolol are mostly attributed to propranolol’s b2 blocking activity and lipophilicity. Theoretically, a solution to these adverse events may be the application of selective hydrophilic b1 blockers, such as atenolol [14,15]. This drug has proven to be effective in the treatment of ulcerated segmental hemangiomas [14,16].
One recent case is of a four-month-old girl put on propranolol 1.5 mg/kg/day for a fast-growing IH of the scalp. Two weeks later, the hemangioma became painful, with wet necrosis and offensive serosanguineous discharge, from which Escherichia coli, Klebsiella pneumoniae, and anaerobes were grown. Tissue debridement revealed a large ulcer, which healed in two weeks. Propranolol was restarted without incident after the healing [17]. The patient also showed a rapid aggravation of the ulceration, which became wide and deep two weeks after the initiation of propranolol. As in the case described, we hypothesize that a rapid involution induced by propranolol could have generated acute intratumoral ischemia responsible for this aggravation, especially since the dose was rapidly increased to 3 mg/kg/day. However, such complications remain rare and propranolol is currently the first-line treatment for ulcerated IH.
Topical treatment for ulcerated IH has been applied, such as timolol alone [18] or in combination with brimonidine solution [19]. This new combination offers synergistic vasoconstriction without additional side effects. It is not recommended, however, to use such a combination in conjunction with additional beta blockers, alpha agonists, or potentiators of these medications, which may pose a risk of serious adverse events [20,21].
The proven efficacy and improved safety profile of propranolol compared to systemic corticosteroids have resulted in the overwhelming adoption of beta blockers in the treatment of complicated IHs. However, the clinical response rate of propranolol as the first-line treatment falls at approx. 90%, and there are still cases with large sizes, serious complications, or low sensitivity to propranolol that cannot achieve a notable improvement after beta-adrenergic receptor antagonists. Therefore, a combination of prednisone and beta-adrenergic receptor antagonists is brought to the forefront by several investigators.
Corticosteroids are known to suppress angiogenesis and vasculogenesis and downregulate vascular endothelial growth factor A (VEGF-A), interleukin-6, monocyte chemoattractant protein-1, and matrix metalloproteinases [22]. However, we cannot specifically explain the effect of rapid ulcer healing that we observed upon the addition of systemic corticosteroids to propranolol. Thus, further studies are required.
Lie et al. [22] consider the addition of oral corticosteroids if significant and painful ulceration persists without notable improvement after two to four weeks. There is no standard protocol for the dose and duration of oral corticosteroid administration, although a prednisolone dose of 1–2 mg/kg/day has been shown to be effective for the treatment of IH [22,23].
Lapidoth et al. [24] showed that eosin inhibits the production of angiopoietin-2 in endothelial cells and may prove efficacious in accelerating the healing of ulcerated hemangiomas of infancy. While eosin has also been shown to bear photodynamic properties, it is unlikely that the photodynamic effect played a role, because the sites were not exposed to therapeutic light sources and were often occluded.
Eosin offers several advantages in terms of treating IHs. First, it has a long record of human safety, including in infants. Second, in addition to being antiangiogenic, it has antibacterial properties. Third and finally, it is less expensive than many other therapies and is therefore affordable in our country.
In an IH, the laser selectively acts on the chromophore, with the oxygen-containing hemoglobin being predominant. The chromophore absorbs light to heat the lesion and causes coagulation, thereby exerting a therapeutic effect [1]. Pulse dye laser is the most commonly used local therapy for ulcerated IHs [7,25]. The combination of pulsed dye laser and the administration of propranolol produces synergistic effects, accelerating the healing of the ulcerated hemangioma [26]. In our case, we were vigilant to avoid alopecia.
CONCLUSION
In this case report, we aimed to present a severe and ulcerated form of segmental hemangioma of the face without associated PHACE syndrome, probably aggravated by a high dose of propranolol.
In our case, combination therapy with propranolol, an oral corticosteroid, and pulsed dye laser with eosin produced good results.
Finally, we hypothesize that a high dose of propranolol in the active phase of a hemangioma may aggravate ulceration and affect the vital prognosis for the young patient, in addition to the scar ratio, which is also aggravated. It is clear, however, that further work is needed to study the relationship between propranolol and the onset or aggravation of ulceration in infantile hemangiomas.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Chen ZY, Wang QN, Zhu YH, Zhou LY, Xu T, He ZY, et al. Progress in the treatment of infantile hemangioma. Ann Transl Med. 2019;7:692.
2. Munden A, Butschek R, Tom WL, Marshall JS, Poeltler DM, Krohne SE, et al. Prospective study of infantile haemangiomas:Incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-13.
3. Yang H, Hu DL, Shu Q, Guo XD. Efficacy and adverse effects of oral propranolol in infantile hemangioma:A meta-analysis of comparative studies. World J Pediatr. 2019;15:546-58.
4. Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
5. Hagen R, Ghareeb E, Jalali O, Zinn Z. Infantile hemangiomas:What have we learned from propranolol?Curr Opin Pediatr. 2018;30:499-504.
6. Hoeger PH, Harper JI, Baselga E, Bonnet D, Boon LM, Ciofi Degli Atti M, et al. Treatment of infantile haemangiomas:Recommendations of a European expert group. Eur J Pediatr. 2015;174:855-65.
7. Wang JY, Ighani A, Ayala AP, Akita S, Lara-Corrales I, Alavi A. Medical, surgical, and wound care management of ulcerated infantile hemangiomas:A systematic review. J Cutan Med Surg. 2018;22:495-504.
8. Léauté-Labrèze C, Prey S, Ezzedine K. Infantile haemangioma:Part II. Risks, complications and treatment. J Eur Acad Dermatol Venereol. 2011;25:1254-60.
9. Hermans DJ, Boezeman JB, Van de Kerkhof PC, Rieu PN, Van der Vleuten CJ, Van der Vleuten CJM. Differences between ulcerated and non-ulcerated hemangiomas, a retrospective study of 465 cases. Eur J Dermatol. 2009;19:152-6.
10. Bay Bay H, Atassi M, Alaoui A, Elfakir S, Kojmane W, Atmani S, et al. Factors related to response to propranolol treatment for infantile hemangiomas:A cross-sectional study on a Moroccan population. Our Dermatol Online. 2020;11:224-30.
11. Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma:A sign of impending ulceration. Arch Dermatol. 2010;146:1235-9.
12. Doulla M, Bako H, Salissou L, Ouédraogo MM, Hassane I, Abdoulaye M, et al. Management of hemangiomas by propranolol:Epidemiological, clinical and therapeutic aspects:retrospective study about 15 cases. Our Dermatol Online. 2018;9:7-10.
13. Solman L, Glover M, Beattie PE, Buckley H, Clark S, Gach JE, et al. Oral propranolol in the treatment of proliferating infantile haemangiomas:British Society for Paediatric Dermatology consensus guidelines. Br J Dermatol. 2018;179:582-9.
14. Wang Q, Xiang B, Chen S, Ji Y. Efficacy and safety of oral atenolol for the treatment of infantile haemangioma:A systematic review. Australas J Dermatol. 2019;60:181-5.
15. Ji Y, Chen S, Wang Q, Xiang B, Xu Z, Zhong L, et al. Intolerable side effects during propranolol therapy for infantile hemangioma:Frequency, risk factors and management. Sci Rep. 2018;8:4264.
16. Ruitenberg G, Young-Afat DA, de Graaf M, Pasmans SGMA, Breugem CC. Ulcerated infantile haemangiomas:the effect of the selective beta-blocker atenolol on wound healing. Br J Dermatol. 2016;175:1357-60.
17. Grech JA, Calleja T, Soler P, Pace D. Necrosis of infantile haemangioma with propranolol therapy. Arch Dis Child. 2020;105:608.
18. Chang CS, Kang GC. Efficacious healing of ulcerated infantile hemangiomas using topical timolol. Plast Reconstr Surg Glob Open. 2016;4:e621.
19. Beal BT, Chu MB, Siegfried EC. Ulcerated infantile hemangioma:Novel treatment with topical brimonidine-timolol. Pediatr Dermatol. 2014;31:754-6.
20. Almebayadh M. Successful treatment of ulcerated infantile hemangioma with brimonidine-timolol cream:2 cases report and review of the literature. J Dermatolog Treat. 2020;31:433-4.
21. Gill K, Bayart C, Desai R, Golden A, Raimer P, Tamburro J. Brimonidine toxicity secondary to topical use for an ulcerated hemangioma. Pediatr Dermatol. 2016;33:e232-4.
22. Lie E, Püttgen KB. Corticosteroids as an adjunct to propranolol for infantile haemangiomas complicated by recalcitrant ulceration. Br J Dermatol. 2017;176:1064-7.
23. Liu C, Zhao ZL, Wu HW, Zheng JW, Wang YA, Liu XJ, et al. Effect of combined low-dose oral prednisone with beta-adrenergic receptor antagonists for refractory infantile hemangiomas:Retrospective cohort study in 76 patients. Ann Transl Med. 2019;7:750.
24. Lapidoth M, Ben-Amitai D, Bhandarkar S, Fried L, Arbiser JL. Efficacy of topical application of eosin for ulcerated hemangiomas. J Am Acad Dermatol. 2009;60:350-1.
25. Chinnadurai S, Sathe NA, Surawicz T. Laser treatment of infantile hemangioma:A systematic review. Lasers Surg Med. 2016;48:221-33.
26. Rodríguez-Ruiz M, Tellado MG, del Pozo Losada J. [Combination of pulsed dye laser and propranolol in the treatment of ulcerated infantile haemangioma]. An Pediatr (Barc). 2016;84:92-6.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0002-5942-441X http://orcid.org/0000-0002-5942-441X |



Comments are closed.