Screening for ocular toxicity of hydroxychloroquine
Florin Maghiar1, Madalina Urma2, Anca Chiriac 3,4, Tudor Pinteala5
3,4, Tudor Pinteala5
1Cardiology Department, Faculty of Medicine and Pharmacy, University of Oradea, Romania, 2”Grigore T Popa”, University of Medicine Iasi, Romania, 3Department of Dermato-Physiology, Apollonia University Iasi, Strada Muzicii nr 2, Iasi-700399, Romania, 4Nicolina Medical Center Iasi, Romania, 5Department of Orthopedics and Traumatology, Faculty of Medicine, Grigore T Popa University of Medicine and Pharmacy, 16 University Street, 7001 Iaşi, Romania
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Hydroxychloroquine (HCQ) is an antimalarial drug often employed in the treatment of various diseases, as a basic treatment for a prolonged period of time.
Materials and Methods: Screening tests were analyzed in descending order of early detection of changes in HCQ toxicity.
Results: The first ocular changes as a result of the toxicity of HCQ are those found during the visual field test, and the typical image of the fundus that highlights the bull’s-eye lesion signifies advanced, irreversible HCQ toxicity.
Conclusion: Ophthalmologic screening is of great importance in daily practice. Physicians from different medical specialties should be aware of the side effects of HCQ and refer the patients to ophthalmology for screening and close follow-up.
Key words: Hydroxychloroquine; Retinal toxicity; Screening
INTRODUCTION
Antimalarial medications (chloroquine and hydroxychloroquine) are derivates of quinine, which were first used in 1630 for the treatment of malaria. Hydroxychloroquine sulfate (HCQ) was synthesized in 1946 and has been widely used as a therapeutic option in various diseases in different medical fields. In the last decades, numerous reports of HCQ administered for skin diseases, psoriatic arthritis and especially rheumatoid arthritis, have been published. Although new drugs are used, HCQ remains a preferred medication and adverse reactions are reported. HCQ is distributed in tissues, yet the lowest concentration was found in the bones, skin, fat tissue, and brain, while higher concentrations were in the muscles, eye, heart, liver, lung, spleen, and adrenal glands, explaining the side effects. The concentration measured in the iris and choroid was 48000 times higher than the plasmatic level of HCQ [1,2].
Systematic ophthalmological screening is recommended when the dose of HCQ is above 200 mg/d [1]. Recently, some risk factors have been studied, such as a high dose, especially in elderly patients or in those with a low BMI or associated comorbidities (retinal disease, renal, or hepatic insufficiency). During the last decade, the risk of retinal toxicity has been evaluated to be 10% at ten years of continuous administration and in a high dose (>5 mg/kg) [2]. Doses >5 mg/kg are not recommended by the American Academy of Ophthalmology [3].
The early stages of toxicity are characterized by abnormalities of retinal pigment epithelium, clearly observed on optical coherence tomography (OCT) by an experienced ophthalmologist. The advanced stages are characterized by bull’s-eye maculopathy [4,5].
Retinal toxicity is irreversible and is determined by the daily dose (recommended: 5 mg/kg; related to the actual weight of the patient) as follows: risk <1% for the first 5 years, 2% for 5–10 years, 20% for over 20 years. The risk of macular toxicity induced by HCQ is considered to begin at a cumulative dose of more than 1000 grams (7 years at a daily dose of around 400 mg/day) [1,6].
Total elimination of HCQ from the body takes over six months with individual variations. The effects of toxicity continue even after the discontinuation of treatment.
MATERIALS
In daily practice, we recommend the following screening tests (in descending order of early detection of HCQ toxicity lesions):
- computerized perimetry, threshold strategy, and macular centering “10-2” (Humphrey Campimeter)/”Macula” (Optopol® Campimeter) (parafoveolar macular centering 100);
- spectral OCT evaluation;
- multifocal electroretinogram;
- fundus autofluorescence.
RESULTS
The first ocular changes as a result of the toxicity of HCQ are those found during the visual field test.
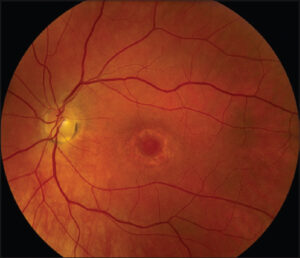
The typical image of the fundus that highlights the bull’s-eye lesion (ang. bull’s-eye retinopathy) signifies advanced, irreversible HCQ toxicity, which should have been prevented by rigorous screening and the discontinuation of the treatment.
The recommended examination strategy is that of threshold 10–2 (points are presented in only 100 paracentral with the removal from the examination of the retinal periphery).
Typically, toxicity first appears by decreasing CV sensitivity in points between 20–80 paracentral (relative to fixation).
Points <20 and >80 are considered to lose sensitivity later, at a higher toxicity of HCQ, and are not involved in the early stages.
The learning curve in CV execution is represented by at least five fields (only after performing at least five fields is the patient sufficiently familiar with the execution technique and rigors, and thus CVs subsequently produced will faithfully represent the functionality of the retina) (Fig. 1).
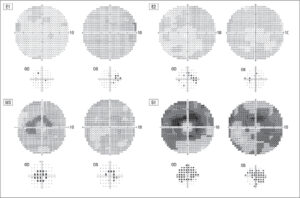 |
Figure 1: General evolution of the visual field in HCQ toxicity. E1, E2: normal/quasi-normal/minimal toxicity; M3, S1: bull’s eye image yet respecting the fovea. |
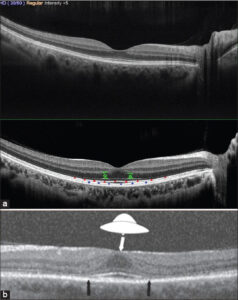
Early OCT signs/landmarks of toxicity (P), before parafoveal ellipsoid zone (ZE) involvement (red landmarks): 1) thinning of the parafoveolar outer nuclear layer (SNE) (green landmarks); 2) reduction of parafoveolar reflectivity (ZE) (red); 3) discontinuities in the appearance of the parafoveolar interdigitated zone (ZI) (blue landmarks).
Fundoscopy is recommended before treatment to identify possible pre-existing retinal pathologies. HCQ toxicity may induce nonspecific changes in the early stages, such as the rarefaction of EPR, loss of the retinal reflex, and discreet pigmentation changes.
Ophthalmologic examination may provide detailed data about HCQ toxicity and the severity of ocular adverse reactions, which may vary from low to moderate and severe (Figs. 2 and 3).
The typical picture of bovine eye retinopathy certifies the severe form of drug toxicity (Fig. 4).
CONCLUSION
It is of huge importance to detect the early modifications due to HCQ to prevent the risk of possible visual loss due to the use of the medication. The immediate interruption of HCQ treatment is recommended when retinal toxicity is suspected. Visual fields and OCT are the most recommended modalities for screening patients treated with HCQ.
Statement of human and animal rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
REFERENCES
1. Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF;American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415-22.
2. Rosenbaum JT, Costenbader KH, Desmarais J, Ginzler EM, Fett N, Goodman SM, et al. American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and American Academy of Ophthalmology 2020 Joint Statement on Hydroxychloroquine Use with Respect to Retinal Toxicity. Arthritis Rheumatol. 2021;73:908-11.
3. Overbury RS, Stoddard GJ, Pupaibool J, Hansen CB, Lebiedz-Odrobina D. The effect of an electronic medical record intervention on hydroxychloroquine prescribing habits and surveyed providers’opinions of the 2016 American Academy of Ophthalmology guidelines in the rheumatology and dermatology practices of an academic institution. BMC Health Serv Res. 2021;21:913.
4. Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye. 2017;31:828-45.
5. Martini L, Brzezinski P. Revisitation of an old antimalarial to combat Psoriatic Arthritis administering an antidote to Santonin to avoid xanthopsia or maculopathy. Our Dermatol Online. 2018;9:269-71.
6. Narváez D, Di Martino Ortiz B, Rodríguez Masi M. Annular skin lesions in childhood:Review of the main differential diagnoses. Our Dermatol Online. 2017;8:75-80.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-0989-4931 http://orcid.org/0000-0002-0989-4931 |





Comments are closed.