Evaluation of the efficacy and safety of the intralesional purified protein derivative of tuberculin versus intralesional vitamin D3 for the management of recalcitrant warts
Manoj Kumar Sharma 1, Alpana Mohta2, Kapil Vyas3, Atul Vijay1
1, Alpana Mohta2, Kapil Vyas3, Atul Vijay1
1Department of Dermatology, Venerology and Leprosy, Jhalawar Medical College, Jhalawar, Rajasthan, India, 2Department of Dermatology, Venerology and Leprosy, Sardar Patel Medical College, Bikaner, Rajasthan, India, 3Department of Dermatology, Venerology and Leprosy, Geetanjali Medical College and Hospital, Udaipur, Rajasthan, India
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Introduction: Warts are caused by the human papillomavirus (HPV), which infects the epidermal cell layers leading to their hyperproliferation. The concept of immunotherapy has recently come to light. It acts by mounting delayed cell-mediated and humoral immunity against the HPV in the host.
Objective: This study aimed to evaluate the efficacy and safety of the intralesional PPD versus intralesional vitamin D3 for the management of recalcitrant warts.
Materials and Methods: This prospective randomized trial was conducted on patients between the ages of 12 and 65 years with two or more recalcitrant extragenital warts. The subjects were randomly divided into two groups, namely, group A (PPD) and group B (vitamin D3). In group A, a purified protein derivative (PPD) of tuberculin was employed without any prior pre-sensitization testing. Each patient in group I received 0.1–0.2 mL of antigen at the base of the largest wart. In group B, the patients received 0.1–0.2 mL in a similar manner.
Results: In group A, 39 (78%) patients had complete clearance while, in group B, 38 (76%) had complete clearance in injected warts (p = 0.8). As far as distant warts were concerned, in group A, 35 (70%) had a complete response while, in group B, 29 (48%) had a complete response. There was a statistically significant difference between the two groups with a greater degree of clearance in distant warts with the tuberculin PPD (p = 0.04).
Conclusion: Both the tuberculin PPD and vitamin D3 are reliable and safe management options for recalcitrant, treatment-resistant, and extensive warts.
Key words: Warts; PPD; Vitamin D3; Immunotherapy
INTRODUCTION
Warts are caused by the human papillomavirus (HPV), which infects the epidermal cell layers leading to their hyperproliferation [1]. Although asymptomatic in most cases, warts tend to spread from one person to another and often pose cosmetic discomfort for the patient. Although a plethora of destructive modalities such as radiofrequency ablation, chemical cauterization, surgical excision, etc., are conventionally utilized for their management, most of these therapies only partially help in extensive and recalcitrant warts. Due to the inherent inability of these management options to mount antiviral immunity in the patient’s body, the risk of recurrence and relapse is high [2,3].
Recently, the concept of immunotherapy has come to light. It acts by mounting delayed cell-mediated and humoral immunity against the HPV in the host. The commonly utilized immunotherapeutic antigens are MMR, BCG, and M. w. vaccines, Candida, Trichophyton, and PPD of tuberculin antigens, vitamin D3, etc. [2,4–8].
The mechanism of action of the PPD vaccine as an immunotherapeutic antigen is the mounting of a Th1-mediated immune response, leading to the upregulation of IL-2,4,5, TNF-a, along with the stimulation of a delayed hypersensitivity reaction [9].
Vitamin D3, on the other hand, has multiple mechanisms of action, namely, increased epidermal cell maturation, reduced cell proliferation, and cytokine production. It acts by downregulating IL-1a and IL-6, upregulating toll-like receptors of human macrophages, and stimulating the formation of the antimicrobial peptide at local and distant sites [10,11].
This study aimed to evaluate the efficacy and safety of intralesional PPD versus intralesional vitamin D3 for the management of recalcitrant warts.
MATERIALS AND METHODS
This prospective randomized trial was conducted after obtaining due approval from the institutional ethics board. The study was conducted between June 2019 and June 2021 in two centers. Immunocompetent patients with recalcitrant verruca were recruited for the dermatology outpatient department.
Inclusion Criteria
Included were patients between the ages of 12 and 65 years with two or more extragenital warts, recalcitrant to at least two conventional modalities attempted in the past. Only patients who had not received any other treatment in the previous two months were included.
Exclusion Criteria
Subjects with both genital and extragenital warts were excluded. Additionally, subjects with any form of congenital, acquired, or iatrogenic immunosuppression, patients with systemic illness, those with a history of hypersensitivity reaction to a PPD, vitamin D3, or any of the additive inactive agents, pregnant or lactating females, and patients with an active infection were excluded.
Detailed records of the patients along with a history of any treatment received in the past were recorded. After explaining the procedure and obtaining written informed consent, the subjects were randomly divided into two groups, namely, group A (PPD) and group B (vitamin D3).
Sample Size
For each group, the sample size was calculated with the probability at 90% and the significant result at 5%. The sample size was fifty per group.
Randomization
In this single-blinded trial, unstratified randomization was performed by an open list of simple, random number tables.
In group A, the purified protein derivation (PPD) of tuberculin was employed without any prior pre-sensitization testing. Each patient in group A received 0.1–0.2 mL of antigen at the base of the largest wart.
In group B, the patients received 0.1–0.2 mL (600,000 IU; 15 mg/mL) of intralesional vitamin D3 (Arachitol-6L®; Akums Drugs and Pharmaceuticals Ltd.) at the base of the largest wart.
In both groups, 0.1–0.2 mL of lignocaine (20 mg/mL) was injected perilesionally beforehand.
Injections were applied every four weeks to the same wart up to four injections or until there was a complete clearance in all warts, whichever happened first. After every session, the results were assessed. Follow-up was performed at six and twelve weeks after the last injection.
Treatment Response
The response to treatment was graded as:
-
• Complete response: clearance in all warts with the appearance of normal skin markings.
-
• Partial response: a 50–99% reduction in the size and number of warts.
-
• No response: a less than 50% reduction in size and number.
Statistical Analysis
Descriptive statistics were performed by calculating the mean and standard deviation for the continuous variables. Categorical variables were presented in the form of absolute numbers and percentages. Nominal categorical data was compared with the chi-square test.
RESULTS
Out of the 115 patients, a hundred completed the study, with fifty patients in each group. Table 1 shows the baseline demographic data of the patients.
In group A, 39 (78%) patients had complete clearance, 5 (10%) had partial clearance, and the remaining 6 (12%) had no clearance in the injected warts (Figs. 1a and 1b). In group B, 38 (76%) subjects had complete clearance, 4 (8%) had partial clearance, and the remaining 8 (16%) had no clearance in the injected warts (Table 2) (Figs. 2a and 2b). There was no significant difference between the two groups (p = 0.81).
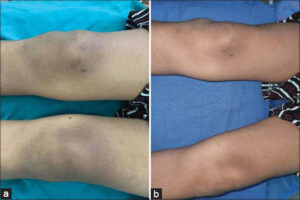 |
Figure 1: (a) Multiple verrucae on the knee at baseline (b) Complete clearance after three sessions of the PPD. |
 |
Table 2: Rate of clearance of the injected warts in the two groups. |
As far as distant warts are concerned, in group A, 35 (70%) had a complete response, 9 (18%) had a partial response, while the remaining 6 had no response. Similarly, in group B, 29 (48%) had a complete response, 5 (10%) had a partial response, while the remaining 16 (32%) had no response (Table 3). There was a statistically significant difference between the two groups, with a greater degree of clearance in distant warts with the tuberculin PPD (p = 0.04) (Table 3).
The mean number of injections required for complete clearance in all warts was 3.1 in group A and 3.7 in group B. In the ensuing twelve weeks of follow-up after the last session, none of the patients experienced a recurrence of their warts.
The side effects were minimal in both groups. The most common adverse event in groups A and B was transient pain on the injection site lasting for 24 hours. Other adverse events were seen only rarely, as transient fever in group A and pruritus on the injection site in group B. No significant correlation could be drawn between the duration of the disease and the treatment response.
DISCUSSION
The treatment of warts is often a therapeutic challenge for the treating dermatologist due to the suboptimal response of the available therapies. Since none of these agents show total effectiveness against HPV, the chances of recurrence are exceedingly high. Due to the presence of HPV in the basal layers of the epidermis, where they often escape the immune system of the body, warts tend to run a chronic and recalcitrant course.
Immunotherapy overcomes the limitation of conventional therapies by stimulating the host’s immunity against HPV. This ability makes this a potent management option for multiple, recalcitrant warts or warts on sites that are difficult to treat, such as genital and periungual [12–14].
Both the PPD of tuberculin and vitamin D3 are widely and easily available and hold promising prospects for managing recalcitrant and multiple warts.
In group A, as far as the injected warts are concerned, a complete response was observed in 39 patients. Due to the obligatory immunization programs of BCG vaccination in India, a majority of the population is already sensitized to mycobacterial antigens. Therefore, there is high PPD immunity in the population. Lahti et al. employed tuberculin jelly as a form of topical immunotherapy for verruca in the year 1982 with a 60% rate of clearance [15]. Abd-Elazeim et al. conducted a study similar to ours and found a 75% clearance rate with the PPD of tuberculin [16]. However, unlike our study, the average number of sessions required for complete clearance was 5.8. A study by Wananukul et al. assessed the efficacy of an intralesional PPD on palmoplantar and periungual warts [17]. They reported complete clearance in 93% of the patients. Kush et al. conducted a similar trial and found a 58.8% improvement in warts [18].
In group B, treated with vitamin D3, among the injected warts, complete clearance was seen in 33 patients and, in distant warts, 29 patients showed complete clearance. There are only a handful of studies on the efficacy of vitamin D3 on warts. The rate of response was reported to range from 40% to 90% [19–26]. The findings of our study were similar to the first study published on the role of intralesional vitamin D3, which was conducted by Aktaş et al. [21]. They reported a complete response in 80% of the patients.
The first studies employing vitamin D3 for managing warts were conducted by Kavya et al. [22] and Raghukumar et al. [23]. They reported a complete response in 78.57% and 90%, respectively. Various authors in the past conducted similar studies to compare the response of intralesional vitamin D3 versus various other immunogens, such as MMR, Candida, and the PPD [19–26]. A study fairly similar to ours was done by Singh et al. [24], who compared the effectiveness of vitamin D3 and the PPD of tuberculin on warts. They found a 72.5% response with vitamin D3. Kareem et al. [26] compared vitamin D3 versus Candida antigen and found a 70% response rate.
The adverse event profile of our patients was minimal, with only slight pain, pruritus, and transient fever reported by less than half of the study subjects. One rare adverse event from vitamin D3 was the formation of granuloma, which resolved on its own in three months. None of the patients treated with the PPD developed flu-like symptoms, vasovagal syncope, or post-inflammatory pigmentation. Similarly, none of the patients in group B developed hypercalcemia. Due to the prior use of local anesthetic, none of the patients developed discomfort while intralesional injections of the antigens were given. Although, in the case of acral sites, vitamin D3 injections tend to cause pruritus and pain more frequently.
Our study was limited by a small sample size, a short follow-up period, and a lack of immunological analysis.
CONCLUSION
Both the PPD of tuberculin and vitamin D3 are reliable and safe management options for recalcitrant, treatment-resistant, and extensive warts. We advocate for these antigens to be employed as the first-line management options for warts.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Mohta A, Kushwaha RK, Agrawal A, Sharma MK, Gautam U, Jain SK. Evaluation of the efficacy of intralesional measles, mumps, and rubella vaccine with intralesional Vitamin D3 as immunotherapies in the treatment of recalcitrant cutaneous warts in adult:A randomized placebo-controlled study. Indian Dermatol Online J. 2021;12:879-87.
2. Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268-71.
3. Braaten KP, Laufer MR. Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Rev Obstet Gynecol. 2008;1:2-10.
4. Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-93.
5. Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts:An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089-93.
6. Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8:209-20.
7. Nofal A, Nofal E. Intralesional immunotherapy of common warts:Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166-70.
8. Maronn M, Salm C, Lyon V, Galbraith S. One-year experience with candida antigen immunotherapy for warts and molluscum. Pediatr Dermatol. 2008;25:189-92.
9. Fathia M. Khattab, Mohamed M. Nasr. A comparative study of topical cantharidin and intralesional PPD to treat molluscum contagiosum. J Dermatol Treat. 2020;31:850-4.
10. Moscarelli L, Annunziata F, Mjeshtri A, Paudice N, Tsalouchos A, Zanazzi M, et al. Successful treatment of refractory wart with a topical activated vitamin D in a renal transplant recipient. Case Rep Transplant. 2011;2011:368623.
11. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-3.
12. Shaldoum DR, Hassan GFR, El-Maadawy EH, El-Maghraby GM. Comparative clinical study of the efficacy of intralesional MMR vaccine vs intralesional vitamin D injection in treatment of warts. J Cosmet Dermatol. 2020;19:2033-40.
13. Fathy G, Sharara MA, Khafagy AH. Intralesional vitamin D3 versus Candida antigen immunotherapy in the treatment of multiple recalcitrant plantar warts:A comparative case-control study. Dermatol Ther. 2019;32:e12997.
14. Garg S, Baveja S. Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg. 2014;7:203-8.
15. Lahti A, Hannuksela M. Topical immunotherapy with tuberculin jelly for common warts. Arch Dermatol Res. 1982;273:153-4.
16. Abd-Elazeim FM, Mohammed GF, Fathy A, Mohamed RW. Evaluation of IL-12 serum level in patients with recalcitrant multiple common warts, treated by intralesional tuberculin antigen. J Dermatolog Treat. 2014;25:264-7.
17. Wananukul S, Chatproedprai S, Kittiratsacha P. Intralesional immunotherapy using tuberculin PPD in the treatment of palmoplantar and periungual warts. Asian Biomedicine. 2009;3:739-43.
18. Kus S, Ergun T, Gun D, Akin O. Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol. 2005;19:515-6.
19. Mohta A, Kushwaha RK, Gautam U, Sharma P, Nyati A, Jain SK. A comparative study of the efficacy and safety of intralesional measles, mumps, and rubella vaccine versus intralesional vitamin D3 for the treatment of warts in children. Pediatr Dermatol. 2020;37:853-9.
20. Shaldoum DR, Hassan GFR, El-Maadawy EH, El-Maghraby GM. Comparative clinical study of the efficacy of intralesional MMR vaccine vs intralesional vitamin D injection in treatment of warts. J Cosmet Dermatol. 2020;19:2033-40.
21. AktaşH, Ergin C, Demir B, Ekiz Ö. Intralesional vitamin D injection may be effective treatment option for warts. J Cutan Med Surg. 2016;20:118-22.
22. Kavya M, Shashikumar BM, Harish MR, Shweta BP. Safety and efficacy of intralesional vitamin D3 in cutaneous warts:An open uncontrolled trial. J Cutan Aesthet Surg. 2017;10:90-4.
23. Raghukumar S, Ravikumar BC, Vinay KN, Suresh MR, Aggarwal A, Yashovardhan DP. Intralesional vitamin D3 injection in treatment of recalcitrant warts:A novel proposition. J Cutan Med Surg. 2017;21:320-4.
24. Singh SK, Mohan A, Gupta AK, Pandey AK. A comparative study between intralesional PPD and vitamin D3 in treatment of viral warts. Int J Res Dermatol. 2018;4:197-201.
25. Abou-Taleb DAE, Abou-Taleb HA, El-Badawy O, Ahmed AO, Hassan AET, Awad SM. Intralesional vitamin D3 versus intralesional purified protein derivative (PPD) in treatment of multiple warts:A comparative clinical and immunological study. Dermatol Ther. 2019;29:e13034.
26. Kareem IMA, Ibrahim IM, Mohammed SFF, Ahmed AA. Effectiveness of intralesional vitamin D3 injection in the treatment of common warts:Single-blinded placebo-controlled study. Dermatol Ther.2019;32:e12882.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-7526-2089 http://orcid.org/0000-0001-7526-2089 |







Comments are closed.