Bullous pemphigoid and the associated co-morbidities: A prospective study at a tertiary-care center
Veerapaneni Vaishnavi , Tambisetti Naresh Babu, Birudala Ramadevi, Bangaru Bhavani Pujitha, Kuna Ramadas
, Tambisetti Naresh Babu, Birudala Ramadevi, Bangaru Bhavani Pujitha, Kuna Ramadas
Department of Dermatology Venereology and Leprosy, Kamineni Academy of Medical Sciences and Research Centre, Hyderabad-500068, India
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Bullous pemphigoid is an autoimmune blistering disorder of the skin. Recent studies have shown that there are various associated co-morbidities existing even before the diagnosis of the condition.
Objectives: The aim was to study the association of co-morbidities in bullous pemphigoid at a tertiary-care center.
Methodology: This was a hospital-based prospective study of forty cases of bullous pemphigoid conducted over a period of thirty months at a tertiary-care center.
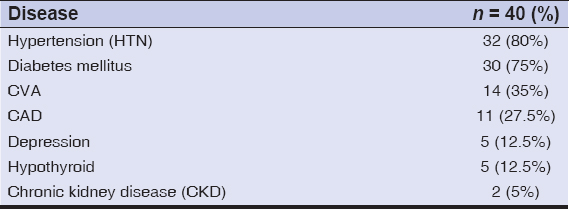
Results: The mean age of the participants was 73. There was a male preponderance, with a male-to-female ratio of 1.8:1. Bullous pemphigoid was significantly associated with hypertension in 80% (32) of the patients, followed by diabetes mellitus in 75% (30), cerebrovascular accidents in 35% (14), coronary artery disease in 27.5% (11), depression in 12.5% (5), hypothyroidism in 12.5 % (5), and chronic kidney disease in 5% (2).
Conclusion: Screening for co-morbid health conditions is paramount in patients with bullous pemphigoid for an optimal outcome since treatment options have an impact on the control of comorbidities and vice versa. It should be a multidisciplinary approach for optimal management and the improvement of the quality of life, thus reducing the number of hospital visits and medications to reduce the economic burden and morbidity, hence improving the quality of life.
Key words: Bullous pemphigoid; Co-morbidities; BPAG1; Hypertension
INTRODUCTION
Bullous pemphigoid (BP) is the most common blistering disorder, predominant among the elderly yet, in rare instances, may affect young adults and children. BP is a disease characterized by the presence of circulating IgG autoantibodies directed against the basement membrane zone. The causative antigens of BP, antigens 1 and 2 (BPAG1 and BPAG2), are detectable by both direct and indirect immunofluorescence [1].
The pathogenesis of BP remains not established with concrete evidence. Some studies have shown that BP has a correlation with co-morbid conditions such as neurological and psychiatric diseases, diabetes mellitus, and malignancies [2–4]. Recently, it has also been observed that the incidence of BP has raised, which could be attributed to factors such as an aging population, an increase in drug-induced cases, and the ameliorated diagnosis of the non-bullous forms [5].
The use of drugs such as dipeptidyl peptidase-4 inhibitors for diabetes mellitus, certain diuretics, antipsychotics, and drugs administered in the treatment of malignancies are also responsible for drug-induced cases [6].
Not only the etiology yet also the presentation of the disease is also quite varied. In the non-bullous phase, patients usually complain of generalized pruritus, erythema, or urticaria-like lesions, whereas, in the bullous phase, they present with tense vesicles or bullae on erythematous or healthy skin appearing symmetrically on the lower trunk, the flexor aspect of the extremities, and the abdomen [7]. The diagnosis of bullous pemphigoid is confirmed by biopsy of the lesions, perilesional direct immunofluorescence, indirect immunofluorescence from the patient’s sera, and immunoblotting [8].
Previously, treating a case of bullous pemphigoid was challenging, as the mortality rate was around 26% [9]. In treating cases of BP, the first line of treatment was systemic and topical corticosteroids. However, the use of systemic corticosteroids produces severe adverse effects, sometimes even leading to death in elderly patients.
Doxycycline, dapsone, methotrexate, azathioprine, mycophenolic acid, intravenous immunoglobulin, rituximab, and omalizumab may be used in patients who cannot tolerate corticosteroids or in refractory cases [10,11]. The study aimed to evaluate demographic and clinical features and the associated comorbidities in cases of BP.
MATERIALS AND METHODS
A hospital-based prospective study was conducted on forty naive cases of BP at a tertiary-care center in South India from July 2019 to December 2021 (thirty months) at the dermatology department. Patients willing to participate in the study and with a histopathological confirmation of BP were included in the study. All cases with other existing autoimmune diseases and neoplasms were excluded.
After taking consent from the patients, data was collected with a pre-designed, semi-structured questionnaire. Data regarding sociodemographic variables and co-existing conditions was collected. Cutaneous and systemic examinations were performed. Histopathological examination and necessary blood investigations related to co-morbidities were conducted. The data was entered and analyzed with Microsoft Excel.
Ethics Statement
An institutional ethical committee certificate was taken.
RESULTS
In this study, the age of the patients ranged between 55 to 80 years, with a mean age of 73 years. A majority were in the 71-80 age group (60%), followed by the 61-70 age group (30%) (Table 1). Out of the forty patients, 65% (26) were male and 35% (14) were female, giving a male-to-female ratio of 1.8:1. All patients had cutaneous blisters (Figs. 1a – 1c) at the time of diagnosis, and 32 (80%) also had urticarial plaques (Fig. 1c). In addition, 3 (7.5%) had the oral mucosa affected and 38 (90%) had pruritus. Peripheral blood eosinophilia was present in 18 (45%) cases.
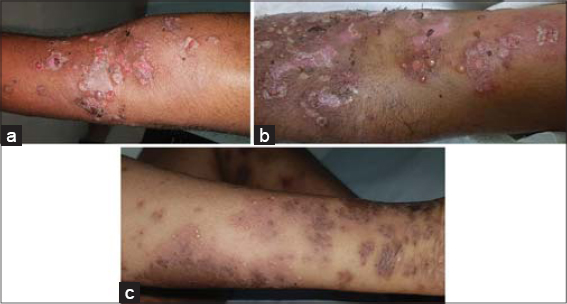 |
Figure 1: Multiple eroded areas and tense bullae on (a) the leg and (b) the hand. (c) Urticarial plaques with multiple blisters on the hand. |
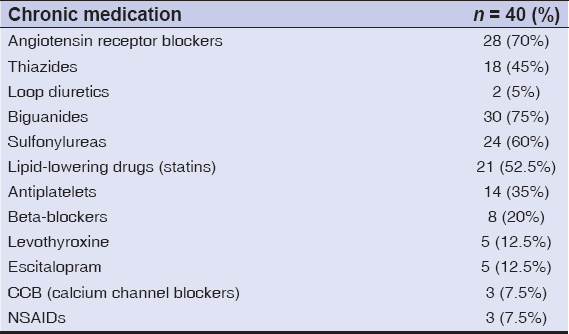
The use of chronic medications (at least 1 drug in the last 6 months) was seen in 37 cases (92.5%), ARBs (angiotensin receptor blockers) in 28 (70%), diuretics in 18 (50%), oral hypoglycemic drugs, biguanides in 30 (75%) and sulfonylureas in 24 (60%), lipid-lowering drugs (statins) in 21 (52.5%), antiplatelets in 14 (35%), beta-blockers in 8 (20%), levothyroxine in 5 (12.5%), escitalopram in 5 (12.5%), CCB (calcium channel blockers) in 3 (7.5%), and NSAIDs in 3 (7.5%) (Table 2).
The major co-morbidities in BP were hypertension in 80% (32) of the cases, diabetes mellitus in 75%(30), cerebrovascular accidents in 35% (14), coronary artery disease in 27.5% (11), depression in 12.5% (5), hypothyroidism in 12.5% (5), and chronic kidney disease in 5% (2) (Table 3).
DISCUSSION
The age of the patients ranged between 55 to 80 years, with a mean age of 73 years. Among the patients, 65% (26) were male and 35% (14) were female, giving a male-to-female ratio of 1.8:1.
In a study conducted by Teixeira et al. [12], the age of the patients ranged from 49 to 96 years. The mean age of the cases was 79.6 ± 8.3 years, and it was in near consonance with our study.
In a study conducted by Askin et al. [13], the age of the patients ranged from 15 to 103 years. The average age at the diagnosis of BP was 73.79 years, and the male-to-female ratio was 1.42:1, which was similar to our study.
However, in a study conducted by Kridin et al. [14], a strong female preponderance among cases of BP was observed, which was a discordant finding in comparison to our study. Most of the existing literature was in support of the finding that BP had a strong female preponderance just as in any other autoimmune disease, yet, in our study, males outnumbered females, which might have been because of a sex-selective and biased approach of the study area, as it was an area set back in a rural, developing part of India.
In our study, the major co-morbidities in bullous pemphigoid were hypertension in 80% (32) of the cases, followed by diabetes mellitus in 75% (30), cerebrovascular accidents in 35% (14), coronary artery disease in 27.5% (11), depression in 12.5% (5), hypothyroidism in 12.5% (5), and chronic kidney disease in 5% (2).
In the study conducted by Askin et al. [13], the most common co-morbidities observed in patients with BP were hypertension in 26 patients (45%), diabetes mellitus in 14 (24%), coronary artery diseases in 6 (1%), chronic kidney disease in 4 (0.7%), and osteoporosis in 4 (0.7%). Hypertension, diabetes mellitus, and coronary artery diseases were common in both sexes. On the other hand, Alzheimer’s disease, osteoporosis, and hypothyroidism were observed more frequently in females; chronic kidney disease was observed more frequently in males.
In our study, the main co-morbidities associated with bullous pemphigoid were hypertension and diabetes, which was in concordance with other studies [15,16]. It has been proposed that an autoimmune response occurs after exposure to BP antigens by the glycation of proteins in the dermoepidermal junction.
In the study conducted by Teixeira et al. [12], a significant association with co-morbidities such as neurological diseases, dementia, a cerebral stroke, Parkinson’s disease, and bed-ridden conditions was observed. This possible association with neurological abnormalities could be considered in line with the hypothesis of immunological cross-reactivity between the neuronal isoform of BPAG1 and its epithelial isoform. Neurological diseases could expose the neuronal isoform and trigger a subsequent immunological reaction causing the cutaneous lesions. Further studies are needed to better understand the underlying molecular pathways.
In a Finnish study conducted by Pankakoski et al. [17], a Finnish cohort of patients with BP was evaluated, with an average age of 77 years, also reporting that the most commonly observed co-morbidities were hypertension (44%), diabetes mellitus (34%), and ischemic heart diseases (26%). A significant association between bullous pemphigoid and a history of malignancies, diabetes mellitus, and chronic obstructive pulmonary disease was found. Furthermore, 46% of the patients had neurologic co-morbidities.
Numerous other studies also observed that cardiovascular conditions such as hypertension were common co-morbidities in cases with BP [18–20].
In our study, it was observed that the use of chronic medications (at least one drug in the last six months) was seen in 37 (92.5%) patients, ARBs (angiotensin receptor blockers) in 28 (70%), diuretics in 18 (50%), oral hypoglycemic drugs, biguanides in 30 (75%) and sulfonylureas in 24 (60%), lipid-lowering drugs (statins) in 21 (52.5%), antiplatelets in 14 (35%), beta-blockers in 8 (20%), levothyroxine in 5 (12.5%), CCB (calcium channel blockers) in 3 (7.5%), escitalopram in 3 (7.5%), fluoxetine in 2 (5%), and NSAIDs in 3 (7.5%).
In the study conducted by Teixeira et al. [12], a strong history of chronic drug use of more than two drugs for various medical conditions was also observed. The drug use profile was also in clear consonance with our findings.
CONCLUSION
Bullous pemphigoid is an autoimmune blistering disease of male predominance usually diagnosed during the sixth and seventh decade of life. Previous studies have shown that bullous pemphigoid is associated with cardiac diseases, diabetes mellitus, and neurologic and psychiatric diseases. Similarly to the literature, our study also showed an association between bullous pemphigoid and cardiac diseases and diabetes mellitus. Bullous pemphigoid was reported to be not associated with malignancies; similarly, our study did not find a significant prevalence of a history of malignant neoplasms in patients with BP, yet an association was found in chronic drug use. Thus, it is advised to elicit a clear history in cases of BP so that the associated gravity of the disease and the morbidity associated may be estimated beforehand, which will help in the further management of the cases.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Abreu Velez AM, Googe, Jr. PB, Howard MS. Immunohistochemistry versus immunofluoresence in the diagnosis of autoimmune blistering diseases. Our Dermatol Online. 2013;4(Suppl.3):585-95.
2. Lai YC, Yew YW, Lambert WC. Bullous pemphigoid and its association with neurological diseases:A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2016;30:2007-15.
3. Belmourida S, Meziane M, Ismaili N, Benzekri L, Senouci K. Unilateral bullous pemphigoid in a hemiplegic patient. Our Dermatol Online. 2022;13:229-30.
4. Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid:A nationwide population-based study. Br J Dermatol. 2011;165:593-9
5. Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. An Bras Dermatol. 2019;94:133-46.
6. Verheyden MJ, Bilgic A, Murrell DF. A systematic review of drug-induced pemphigoid. Acta Derm Venereol. 2020;100:adv00224.
7. Schmidt E, della Torre R, Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Immunol Allergy Clin North Am. 2012;32:217-32.
8. Ujiie H, Nishie W, Shimizu H. Pathogenesis of bullous pemphigoid. Dermatol Clin 2011;29:439-46, ix.
9. Liu YD, Wang YH, Ye YC, Zhao WL, Li L. Prognostic factors for mortality in patients with bullous pemphigoid:A meta-analysis. Arch Dermatol Res. 2017;309:335-47.
10. Ruggiero A, Megna M, Villani A, Comune R, Fabbrocini G, di Vico F. Strategies to improve outcomes of bullous pemphigoid:A comprehensive review of clinical presentations, diagnosis, and patients’assessment. Clin Cosmet Investig Dermatol. 2022;15:661-73.
11. Di Lernia V, Casanova DM, Goldust M, Ricci C. Pemphigus vulgaris and bullous pemphigoid:Update on diagnosis and treatment. Dermatol Pract Concept. 2020;10:e2020050.
12. Teixeira VB, Cabral R, Brites MM, Vieira R, Figueiredo A. Bullous pemphigoid and comorbidities:A case-control study in Portuguese patients. Anais brasileiros de dermatologia. 2014;89:274-8.
13. Aşkin Ö, Özkoca D, Uzunçakmak TK, Mat C, Kutlubay Z. Epidemiology and comorbidities of bullous pemphigoid:A retrospective study. J Turk Acad Dermatol. 2020;14:53.
14. Kridin K. Subepidermal autoimmune bullous diseases:Overview, epidemiology, and associations. Immunol Resvol. 2018;66:6-17.
15. Sánchez-García V, Pérez-Alcaraz L, Belinchón-Romero I, Ramos-Rincón JM. Comorbidities in patients with autoimmune bullous disorders:Hospital-based registry study. Life 2022;12:595.
16. Titou H, Kerrouch H, Frikh R, Hjira N. The association between bullous pemphigoid and comorbidities:A case-control study in Moroccan patients. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:7-11.
17. Kulthanan K, Chularojanamontri L, Tuchinda P, Sirikudta W, Pinkaew S. Prevalence and clinical features of Thai patients with bullous pemphigoid. Asian Pac J Allergy Immunol. 2011;29:66-72.
18. Pankakoski A, Sintonen H, Ranki A, Kluger N. Comorbidities of bullous pemphigoid in a Finnish cohort. Eur J Dermatol 2018;28:157-61.
19. Atzmony L, Mimouni I, Reiter O, Leshem YA, Taha O, Gdalevich M, Hodak E, Mimouni D. Association of bullous pemphigoid with malignancy:A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:691-9.
20. Bech R, Kibsgaard L, Vestergaard C. Comorbidities and treatment strategies in bullous pemphigoid:An appraisal of the existing literature. Front Med (Lausanne). 2018;5:238.
Notes
Source of Support: Nil,
Conflict of Interest: The authors have no conflict of interest to declare.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-5759-9868 http://orcid.org/0000-0001-5759-9868 |



Comments are closed.