Effectiveness of the active ingredients (Capixyl, Procapil, and rosemary extract) of the Trust® tonic for the treatment of androgenetic alopecia in comparison to minoxidil
Ehsan Eslahi , Nooshin Hashemi, Sara Shamaei
, Nooshin Hashemi, Sara Shamaei
The R&D Department, Mahloran Cosmetic Company, Tehran, Iran
Citation tools:
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Androgenic alopecia (AGA) is a common hair loss disorder seen in both males and females and continues by presenting thinning and miniaturization because of androgens, genetics, extracellular matrix (ECM) protein destruction, and micro-inflammation. The most common treatments for AGA used in males are minoxidil and finasteride. These drugs have an effective role in the recovery and retardation of hair loss; however, there are different side effects and limitations to their efficacy.
Materials and Methods: In this research, we compared the effectiveness of the Trust tonic’s active complex, Capixyl, Procapil, and rosemary extract (CPR), and 2% minoxidil solution in reducing hair loss and stimulating hair growth. The study was conducted on two groups of male subjects with an average of 45 years for twenty-four weeks using 1 ml of each solution everyday in the morning and evening.
Results: The results of this study revealed that the subjects treated with the Trust active complex, obtain significantly higher self-assessment (64% in the Trust tonic group and 36% in the minoxidil group) and the staff assessment scores of hair growth improvement in 60% and 30%, respectively, for the Trust tonic group and minoxidil group. Furthermore, the scores obtained by the photographic method also revealed a 57% and 8% improvement in hair growth in the patients who used the Trust complex and minoxidil, respectively.
Conclusions: The active complex of the Trust tonic solution could be an effective alternative for minoxidil in the treatment of AGA.
Key words: androgenic alopecia; Trust tonic; minoxidil; hair growth
INTRODUCTION
Hair loss is the transformation of terminal hair into vellus hair (thin, short, light-colored hair that usually goes where it naturally lacks hair) [1–3]. These conditions occur gradually and to varying degrees in both males and females. The most generic form of hair loss has a genetic origin and is related to androgens (steroid hormones responsible for secondary traits) [4–7]. Thus, androgenic baldness, or common hair loss, is a natural phenomenon related to aging and occurs in both sexes [2,8]. Studies have revealed that mammalian hair follicles have three growth phases: the anagen (growth), catagen (transfer), and telogen (rest) [9,10]. The duration of each phase varies depending on the person’s anatomy, nutritional and hormonal status, and age. More than forty years ago, Hamilton found that androgens were the most crucial factor in male-pattern hair loss [11]. Testosterone is an androgen produced by the gonads and adrenals and plays a vital role in controlling human hair growth. The pattern of hair loss is more complex for females than for males. Therefore, the term male-pattern baldness is employed for patterns of baldness that begin at the crown of the head and move forward or begin at the forehead and move backward until a pattern of baldness is created. Post-menopausal females show this pattern of male baldness [12]. The main cause of hair loss, despite various stimulants, is the shortening of the anagen to the telogen phase of hair growth. As the balding process progresses, the ratio of anagen to telogen hair decreases [13]. There are common and effective treatments for all types of hair loss. The use of these compounds may prevent hair loss or reduce the rate of hair loss. The common treatments for hair loss include herbal supplements reducing or preventing hair loss orally, topically, and surgically [14]. For instance, the combination of biochanin A, acetyl tetrapeptide-3, and ginseng extract versus 3% minoxidil was employed in the latest research for the potential treatment of AGA by decreasing side effects and increasing patient adherence to this complex [15]. These herbal extracts in a combination with peptides created an increase in hair matrix proteins such as collagen and laminin, strengthening hair anchoring, hair growth, and hair follicle size [16]. The common treatments for age-related hair loss include minoxidil, finasteride, and others. Minoxidil is an over-the-counter drug that helps hair regrowth and slows down hair loss, yet it has side effects such as inflammation and itching of the scalp and the growth of unwanted hairs on the scalp, face, and areas adjacent to the treated area. Finasteride is also a prescription drug prescribed as oral pills. Similarly, this compound promotes new hair growth and slows down hair loss six months after administration. Nowadays, however, due to the side effects related to these two drugs, other effective actives have been employed to treat AGA. For this purpose, we evaluated the efficacy of the three active compounds (Capixyl, Procapil, and rosemary extract, called the CPR) employed simultaneously in the formulation of Trust tonic solution as a beneficial complex for the treatment of androgenic alopecia in comparison to 2% minoxidil.
MATERIALS AND METHODS
Materials used to formulate the active treatment solutions comprise all the active ingredients and the basic ingredients of a formulation. The Trust’s tonic solution formulated by the Mahloran Cosmetics company prepared with the active ingredients of Capixyl, obtained by the Lucas Meyer Cosmetics company (Canada), in 1% w/w, Procapil, obtained by the Sederma company (France), in 1%, and rosemary extract, prepared by the Giah Essence Phytopharma company, in 0.5%. Other basic ingredients, as well as water, are added to reach 100% of w/w. Furthermore, the minoxidil compound in the concentration of 2% was purchased from a local drug store in IRAN. All devices used in this study included a pH meter (Benchtop Meter, PH500, CLEAN, China), a digital scale (PS 600.R2, RADWAG, Poland), a beaker, a mixer (MS 280D, MTOPS, Korea), and a heater-stirrer (MS300HS, MTOPS, Korea).
In this study, two groups of twenty healthy males with an average age of 45 years were selected. All participants had stage II-V androgenic alopecia based on the Norwood-Hamilton classification (Table 1) [17,18]. Patients with dermatological disorders such as lung disease or cardiovascular, liver, or kidney disorders and patients on steroids, antihypertensives, cyclosporine, beta-blockers, or antidepressants [19] were excluded from the research. All clinical investigations were conducted according to the principles of the Declaration of Helsinki. All subjects were trained to use the test compounds and instructed in the topical use of these compounds on hairless areas. All subjects were asked to cut their hair to the same length and form, use the same shampoo, and standardize all variables during the treatment period. The subjects were requested to use the Trust tonic active ingredients (Capixyl, Procapil, and rosemary extract) in one group and minoxidil as the comparative active in the other group. These compounds were applied to the hair everyday in the morning and evening with the fingertips in 1 mL of volume each time for a period of twenty-four weeks. The subjects in both groups were unaware of the materials employed in the formulation of the treatment compounds, and the numbered solutions provided them with no clues.
Efficacy Measurements
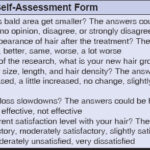
After preparing the subjects and solutions, they were asked to answer the questions (Table 2). This questionnaire regarded the self-assessment of hair density, hair growth and length, the hair loss rate, and satisfaction after every four weeks. Finally, each subject informed the researchers about their satisfaction with a rating scale varying from -3 to +3.
In addition, the researcher staff assessed the efficacy of the formulation and amount of hair growth and density in the hairless area with seven-point scaling criteria, with +3 meaning improvement to -3 meaning worsening (Table 3).
 |
Table 3: Standardized rating scale for the assessment of our subjects |
Also, a global photographic evaluation with a seven-point scale was employed to compare the top and front area of the head skin before and after 24 weeks of administering the Trust tonic and minoxidil topically. For this purpose, some pictures were taken from the front and top of the hair of all subjects including the group treated with the Trust tonic and the group treated with minoxidil before and after the treatment. Then, the growth rate and the efficacy of the Trust tonic were compared with minoxidil and the results were reported.
Ethics Statement
This manuscript describes recent work and is not under consideration for publication by any other journal. All authors approved the manuscript and this submission.
RESULTS
The primary aim of this study was to assess the effectiveness of the Trust tonic’s active ingredients (Capixyl, Procapil, and rosemary extract) in decreasing or eliminating hair loss as well as improving or increasing hair growth and its quality. Thus, a study was conducted on two groups of males with an average age of 45 with the Trust tonic’s active ingredients and minoxidil in each group, respectively. During the study, the effectiveness of these compounds on hair condition and its changes were assessed with a seven-point, photographic evaluation, a seven-point, self- and staff-assessment scale. According to the data collected in this study, the active complex of the Trust tonic and minoxidil both demonstrated a positive trend in hair growth and condition, yet the efficacy of the Trust tonic was significantly greater than that of minoxidil. These results are shown in the parts following below.
Staff-Evaluation
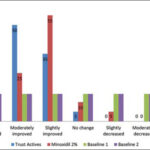
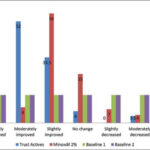
Among the forty individuals who had participated in this research, all completed the study. Based on staff evaluation and rating scores, the assessment revealed better recovery and improvement as a greatly improved label in the group treated with the Trust active complex (10%) than in the group treated with minoxidil (5%), and as a moderately improved label in the group treated with the Trust tonic active ingredients (50%) when compared to minoxidil (25%) (Fig. 1).
Self-Evaluation
The evaluation of the self-scoring form and all of the subjects’ responses indicated that the subjects in the group treated with the Trust tonic and the subjects in the group treated with 2% minoxidil answered the first question in Table 2 as strongly agree and agree, which was statistically significant (p < 0.05) (Table 4).
| Table 4: Self-evaluation score of hair appearance after treatment with the Trust tonic and minoxidil in both groups |
Also, the subjects treated with the Trust tonic displayed a greater improvement in the appearance of the head skin in the bald areas. They stated that the topical use of the Trust tonic had impressive effects on parameters such as hair growth, hair length, and hair density, and they were satisfied with the results of hair loss retardation (Tables 5-7).
Photographic Scoring
In all subjects with AGA, the evaluation of hair growth and of the appearance of the bald areas was done with a photographic method in both groups. The global photographic assay revealed a significantly greater improvement in hair growth in the patients treated with the Trust tonic in comparison to the group treated with minoxidil (Fig. 2).
DISCUSSION
As described before, hair loss is a common concern seen in 50% of males under their fifties [20], and is known as androgenic alopecia (AGA). The conversion of testosterone to dihydrotestosterone by the 5a-reductase enzyme is the main trigger of AGA. In this situation, the hair follicle miniaturizes and falls out. Depending on the severity of hair loss and hormonal disorders, different types of AGA are seen in the frontotemporal regions and crown of the head [21]. Much research has been done to stop or retard hair loss in patients suffering from AGA. For this reason, minoxidil and oral finasteride are the two common drugs approved by the FDA to regulate the biological responses and hinder hair loss. Although these drugs have been accepted to be applied as treatments for AGA, they produce several topical and systemic side effects [22]. For instance, the growth of unwanted hairs, burning, inflammation, and irritation or allergic contact dermatitis of the head skin in the applied area, and a faster heart rate are the main topical and systemic complications of minoxidil, respectively [23]. Thus, other active compounds as safer and more effective alternatives for these two compounds have been investigated. Capixyl is an innovative complex of biomimetic peptide (acetyl tetrapeptide-3) combined with the red clover extract (Trifolium pratense) employed as one of the main active ingredients in the formulation of the Trust tonic. One of the main effects of Capixyl is an increase in hair density and width [24]. This extract is enriched with the isoflavone biochanin A, which is a powerful inhibitor of 5a-reductase type I and II enzymes [25,26]. Also, based on other research studies, biochanin A strongly suppresses these two isoforms of the 5a-reductase enzyme found in the scalp, especially the type II isoform that contributes to AGA [27]. In the case of peptides, recent studies have shown that the size of hair follicles is determined by the number of cells in the dermal papilla and the volume of extracellular matrix proteins (ECM). These proteins express collagen type I, III, and VII and laminins, which have a particular role in maintaining the hair follicle and the dermal papilla’s cell proliferation and differentiation [28, 29]. Hence, these biomimetic peptides employed in this study in combination with the red clover extract based on former research, are shown to produce a significant effect on the synthesis of ECM proteins and further improve the hair morphology and growth via the strengthening the hair follicle anchorage [24]. Same as Capixyl, Procapil is another important active ingredient applied in the formulation of the Trust tonic to improve hair growth and retarding hair loss in cases with complaints of AGA on various levels. Procapil is a vitaminized complex of apigenin and oleanolic acid. Apigenin derived from the citrus peel has a vasodilation effect, increasing scalp microcirculation and hindering follicle aging and premature hair loss. Oleanolic acid extracted from the olive tree leaves is a potent inhibitor of 5a-reductase I and II enzymes and plays a key role in follicle atrophy by preventing dihydrotestosterone production. The third part of this effective anti-hair loss ingredient is a vitaminized peptide that encompasses glycine-histidine-lysine peptides essential for the metabolic activity of hair follicles [19].
Rosemary extract from the Rosmarinus officinalis herb is employed as one of the active complexes in the Trust tonic and is another innovative active compound that acts as an antibacterial, antifungal, anti-inflammatory, and antioxidant compound [30]. Rosemary was used as an enhancer of microcapillary perfusion in 100 patients with AGA in a concentration of 3.7 mg/mL in a recent comparative study on two groups with 2% minoxidil. The results revealed that the hair count increased in both groups, yet the common adverse effect of itching related to minoxidil was reported in the group treated with minoxidil and not in those taking rosemary extract. Thus, as a natural and safe ingredient, rosemary extract could be used as an alternative option for minoxidil in patients with AGA [31,32].
In this study, we evaluated the effectiveness of the Trust tonic’s active ingredients in a concentration of 2.5% as an alternative to 2% minoxidil, which is known as an accepted drug for the treatment of AGA. The researchers’ scores on the evaluation of hair growth revealed that the maximum hair growth was seen in the patients treated with the Trust tonic (60%: greatly and moderately improved; p < 0.05) in comparison to the patients treated with minoxidil (30%: greatly and moderately improved; p < 0.05) after the 24 weeks of treatment. Hence, hair growth improvement in the group on the Trust tonic was five times greater than in the group on minoxidil, which was significant statistically. In the self-assessment tests, results in both groups using the Trust tonic and minoxidil showed the efficacy and satisfaction of the patients in the group using the Trust tonic than the group using minoxidil. Based on the patients’ scores, the appearance of hair after the Trust tonic and minoxidil scored much better and somewhat better in the average of 68.75% and 31.25%, respectively. Furthermore, the results of self-evaluation of scalp baldness revealed a decrease of 56.25% and 43.75% in the subjects on the Trust tonic and minoxidil, respectively. Also, hair growth and the size and density of the hair in the patients treated with the Trust tonic represented an averagely perfect and moderate increase of 64%, which was significantly higher in the patients treated with minoxidil (36%). The effectiveness of the Trust tonic in decreasing hair loss showed a score of 57.69% (effective and highly effective) on average, which was more significant in the group on minoxidil (42.30%).
The photographic evaluation of the head scalp revealed a significant improvement (great, moderate, and slightly improved) in the group treated with the Trust tonic compared to the group treated with minoxidil (90.5% in the Trust tonic group and 64% in the minoxidil group; p < 0.05) (Fig. 2). Therefore, the Trust active complex showed a 1.4 times higher recovery rate in the patient’s hair count and appearance and could be a fair substitute for minoxidil in patients suffering from AGA.
CONCLUSION
Natural compounds such as herbal extracts, antioxidants, and vitamins may prevent androgenic alopecia by radical scavenging activity, hindering inflammation, and increasing the volume of extracellular matrix proteins (ECM) in the hair follicle area. Thus, this research focused on novel complexes in the treatment of androgenic alopecia and receding hair loss. Using these effective compounds in combination could be an effective alternative for minoxidil in the treatment of patients with AGA.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Sinclair R. Male pattern androgenetic alopecia. Bmj. 1998 Sep 26;317:865-9.
2. Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89-95.
3. Lueangarun S, Panchaprateep R. An herbal extract combination (biochanin A, acetyl tetrapeptide-3, and ginseng extracts) versus 3% minoxidil solution for the treatment of androgenetic alopecia:A 24-week, prospective, randomized, triple-blind, controlled trial. The Journal of clinical and aesthetic dermatology. 2020;13:32.
4. Obana NJ, Uno H. Dermal papilla cells in macaque alopecia trigger a testosterone-dependent inhibition of follicular cell proliferation, in van Neste D, Randal VA (eds):Hair research in the next millenium, Elsevier, Amsterdam. 1966;307-10.
5. Kaufman KD. Androgen metabolism as it affects hair growth in androgenetic alopecia, in Whiting DA (ed):Update in Hair Disorders, Dermatol Clin, 14:697-711, 1996.
6. Khandpur S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol. 2002;29:489-98.
7. Hamilton JB. Male hormone stimulation is prerequisite and an incitant in common baldness. Am J Anat. 1942;71:451-80.
8. Dhariwala MY, Ravikumar P. An overview of herbal alternatives in androgenetic alopecia. J Cosmet Dermatol. 2019;18:966-75.
9. Lourith N, Kanlayavattanakul M. Hair loss and herbs for treatment. J Cosmet Dermatol. 2013;12:210-22.
10. Bernard BA. La vie révélée du follicule de cheveu humain. Medecine/Sciences. 2006;22:138-43.
11. Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert opinion on pharmacotherapy. 2010;11:1295-304.
12. Ho CH, Sood T, Zito PM. Androgenetic alopecia. InStatPearls Internet 2021 May 5. StatPearls Publishing.
13. Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen effluvium:A review of the literature. Cureus. 2020;12.
14. Hosking AM, Juhasz M, Mesinkovska NA. Complementary and alternative treatments for alopecia:A comprehensive review. Skin Appendage Disord. 2019;5:72-89.
15. Lueangarun S, Panchaprateep R. An herbal extract combination (biochanin A, acetyl tetrapeptide-3, and ginseng extracts) versus 3% minoxidil solution for the treatment of androgenetic alopecia:A 24-week, prospective, randomized, triple-blind, controlled trial. J Clin Aesthet Dermatol. 2020;13:32.
16. Loing E, Lachance R, Ollier V, Hocquaux M. A new strategy to modulate alopecia using a combination of two specific and unique ingredients. J Cosmet Sci. 2013;64:45-58.
17. Norwood OT. Male pattern baldness:Classification and incidence. South Med J. 1975;68:1359-65.
18. Gupta M, Mysore V. Classifications of patterned hair loss:A review. J Cutan Aesthet Surg. 2016;9:3.
19. Karaca N, Akpolat ND. A comparative study between topical 5% minoxidil and topical Redensyl, Capixyl, and Procapil combination in men with androgenetic alopecia. J Cosmetol Trichol. 2019;150370987.
20. Shapiro J, Wiseman M, Lui H. Practical management of hair loss. Can Fam Physician. 2000;46:1469-77.
21. Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010;11:1295-304.
22. Headington JT. Hair follicle biology and topical minoxidil:Possible mechanisms of action. Dermatol. 1987;175(Suppl. 2):19-22.
23. Habibullah Aktas SA, Türkoglu EB, Sevik Ö. Could topical minoxidil cause non-arteritic anterior ischemic optic neuropathy? J Clin Diagn Res. 2016;10:WD01.
24. Loing E, Lachance R, Ollier V, Hocquaux M. A new strategy to modulate alopecia using a combination of two specific and unique ingredients. J Cosmet Sci. 2013;64:45-58.
25. Booth NL, Piersen CE, Banuvar S, Geller SE, Shulman LP, Farnsworth NR. Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause:A literature review. Menopause. 2006;13:251-64.
26. Evans BA, Griffiths K, Morton MS. Inhibition of 5a-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J Endocrinol. 1995;147:295-302.
27. Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS, Dutasteride Alopecia Research Team. The importance of dual 5a-reductase inhibition in the treatment of male pattern hair loss:Results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-23.
28. Messenger AG, Elliott K, Temple A, Randall VA. Expression of basement membrane proteins and interstitial collagens in dermal papillae of human hair follicles. J Investig Dermatol. 1991;96:93-7.
29. Aumailley M, Rousselle P. Laminins of the dermo-epidermal junction. Matrix Biol. 1999;18:19-28.
30. Albuquerque TG, Castilho MC, Ramos F, Melo NR, Sanches-Silva A. A novel insight on an ancient aromatic plant:The rosemary (Rosmarinus officinalis L.). Trends Food Sci Tech. 2015;45:355-68.
31. Panahi Y, Taghizadeh M, Marzony ET, Sahebkar A. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia:A randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hosking AM, Juhasz M, Mesinkovska NA. Complementary and alternative treatments for alopecia:A comprehensive review. Skin Appendage Disord. 2019;5:72-89.
Notes
Source of Support: Nil,
Conflict of Interest: The authors have no conflict of interest to declare.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-6330-2856 http://orcid.org/0000-0001-6330-2856 |







Comments are closed.