a–m
Cardiovascular comorbidities in patients with a severe form of psoriasis: A case-controlled study assessing clinical, biochemical, and radiological parameters
Samagani Akshay , Yadalla Kumar Harikishan
, Yadalla Kumar Harikishan
Department of Dermatology, Venereology and Leprosy, Raja Rajeswari Medical College & Hospital, Bangalore, India
Corresponding author: Samagani Akshay, MD
Submission: 30.11.2021; Acceptance: 16.02.2022
DOI: 10.7241/ourd.20223.2
Cite this article: Akshay S, Harikishan YK. Cardiovascular comorbidities in patients with a severe form of psoriasis: A case-controlled study assessing clinical, biochemical, and radiological parameters. Our Dermatol Online. 2022;13(3):248-253.
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Psoriasis is a common, chronic, inflammatory, and proliferative disorder of the skin, and is associated with elevated lipid levels and exaggerated inflammatory status, which may lead to cardiovascular morbidities and mortalities.
Aims: The aim was to estimate the cardiovascular risk profile among psoriatic patients when compared to controls.
Settings and Design: This was a case-controlled study performed at a tertiary-care hospital.
Methods and Material: One hundred patients with severe psoriasis were evaluated for clinical, biochemical, electrocardiographic, and radiological signs of cardiovascular comorbidities and were compared with one hundred age- and sex-matched controls.
Statistical Analysis: IBM Corp. Released 2015. IBM SPSS Statistics for Windows, version 23.0. Armonk, NY: IBM Corp.
Results: Males predominated (1.27:1), with the most common age group being 31 to 40 years old. Smoking and alcohol consumption were common among the psoriatic patients (odds ratio = 4.14 and 2.38, respectively). The mean PASI score among the cases was 26.19 – 10.8, with an extreme effect on their quality of life. The psoriatic patients presented with independent cardiovascular risk factors, including systolic hypertension (148.67 ± 10.91 mm of Hg), elevated fasting blood sugars (129.46 ± 35.64 mg/dL), hypercholesterolemia (152.34 ± 44.56 mg/dL), and hypertriglyceridemia (186.29 ± 29.45 mg/dL), with metabolic syndrome among 41% of the cases. On the electrocardiogram, the P wave was elevated (22%) and the QTc interval was prolonged (20%) among the cases. On high-resolution ultrasonography, the intima-media of the carotid arteries were thickened (0.944 ± 0.132 mm), indicating subclinical atherosclerosis in the psoriatic patients.
Conclusions: Early detection and periodic screening for cardiovascular risk factors among psoriatic patients and aggressive therapeutic management are recommended to reduce the disease burden and improve their quality of life.
Key words: Cardiovascular comorbidities; Carotid-artery intima–media thickness; Electrocardiogram; Major adverse cardiovascular events; Psoriasis
INTRODUCTION
Psoriasis is a genetically predisposed, environmentally triggered, chronic, inflammatory, and proliferative dermatosis affecting the skin and beyond [1]. Globally, more than 125 million individuals are affected, among which one third presents with moderate to severe forms of the disease [2]. Approx. 25 million individuals are affected by psoriasis in India, accounting for 20% of the global disease burden, with the prevalence varying from 0.8-5.6% [3]. Psoriasis is associated with systemic manifestations such as psoriatic arthritis, Crohn’s disease, ulcerative colitis, depression, hypertension, diabetes, dyslipidemia, metabolic syndrome, and cardiovascular diseases, such as coronary artery calcification and myocardial infarction [4]. An increased risk of peripheral vascular disease, atrial fibrillation, and venous thromboembolism has been demonstrated [5].
Psoriasis, being a Th1/17-dependent inflammatory skin disease, has been associated with both cutaneous and systemic manifestations and shares a common pathway of systemic inflammation with a deranged lipid profile and cardiovascular diseases [6,7]. Emotional stress, alcohol use, smoking, a high-calorie diet, and obesity produce deleterious effects on inflammatory conditions such as psoriasis and major adverse cardiovascular events (MACEs) [8]. The severe form of psoriasis and its associated comorbid conditions have a negative impact on the overall quality of life for psoriatic patients. The mortality rate is higher in patients with moderate-to-severe psoriasis when compared to healthy controls [9,10]. The life expectancy of patients is decreased by approx. five years, mainly due to cardiovascular comorbidities [11]. Furthermore, the association of cardiovascular comorbidities among patients with psoriasis has a significant impact on the healthcare system [12,13].
Our objective was to compare the cardiovascular risk profile of patients with severe psoriasis to controls without psoriasis.
MATERIALS AND METHODS
The present study was a hospital-based, case-controlled study conducted at a tertiary-care center in a semi-urban region of south India.
One hundred clinically diagnosed psoriatic patients with a severe form of the disease, aged eighteen years and above, belonging to both sexes, and consenting for the study, were evaluated for cardiovascular risk factors. They were compared against normal controls of a similar age group and sex, and selected by a systemic sampling method. Patients with mild and moderate forms of psoriasis or the severe disease on active therapeutic management within the last four weeks, a known case of cardiovascular disease and/or any other systemic illness, psychiatric patients, and pregnant women were excluded from the study.
Cases were evaluated by obtaining a detailed history (smoking, alcohol use, etc.) and a thorough clinical examination. To assess the severity of the disease, the Psoriasis Area Severity Index (PASI), a worksheet by the British Association of Dermatologists, was employed, with scores ranging from 0 to 72 and with the severe form of the disease qualified by a score ≥ 10 [14]. The disease effect on the quality of life (severity score > 10) was assessed by the Dermatology Life Quality Index (DLQI). A self-assessment sheet formulated by Cardiff University, was employed after obtaining the necessary permissions [15]. Clinical parameters, including systolic (SBP) and diastolic blood pressures (DBP), height, weight, and waist circumference, were measured. The body mass index (BMI) was calculated as per criteria by the National Cholesterol Education Program, Adult Treatment Panel III.
Blood tests, including the erythrocyte sedimentation rate (ESR), fasting blood sugars (FBS), and a lipid profile, were performed and compared with controls. Serum total cholesterol (TC) and triglycerides (TG) were determined by the enzymatic method. Serum high-density lipoproteins (HDL) were estimated by the phosphotungstate method. The serum level of very-low-density lipoproteins (VLDL) was calculated by the formula VLDL = TG/5. The serum level of low-density lipoproteins (LDL) was calculated by the Friedewald equation, or by the direct enzymatic method if the values were above 400 mg/dL.
The metabolic syndrome was diagnosed on the basis of criteria by the National Cholesterol Education Program, Adult Treatment Panel III (Table 1).
 |
Table 1: Risk factors for metabolic syndrome (NCEP-ATP III) |
A twelve-lead electrocardiogram (ECG) was obtained by an ultra-light, single-channel ECG machine (Cardiart 6108T, BPL). The carotid artery intima-media thickness (CIMT) was measured by the high-resolution ultrasonography method with Samsung RS80A, a linear probe (LA3-16A), and a frequency of 12 Mhz.
Statistical Analysis
All demographic parameters and categorical and numerical data were tabulated on an Excel sheet. Statistical analysis was performed with SPSS Statistics for Windows, version 23.0 (Armonk, NY: IBM Corp.). Results on the continuous measurements were presented as means ± standard deviations, and results on the categorical measurements were presented as numbers or percentages. The analysis of variance (ANOVA) was employed to find the significance of the study parameters between the groups of patients. The Fisher’s exact test and odds ratios (OR) were used to find the significance of the study parameters on the categorical scale between two or more groups, with a confidence interval of 95% and a p value below 0.05 considered significant.
Ethics Statement
The approval of the institutional ethics committee was obtained for our study (IEC registration no.: XXMCH-IEC/70/2018-19).
RESULTS
The cases and controls were the residents of a semiurban region in south India, with a majority belonging to low socioeconomic status. The study variables with respect to cases and controls and the association of risk factors to psoriasis are discussed in Tables 2 and 3.
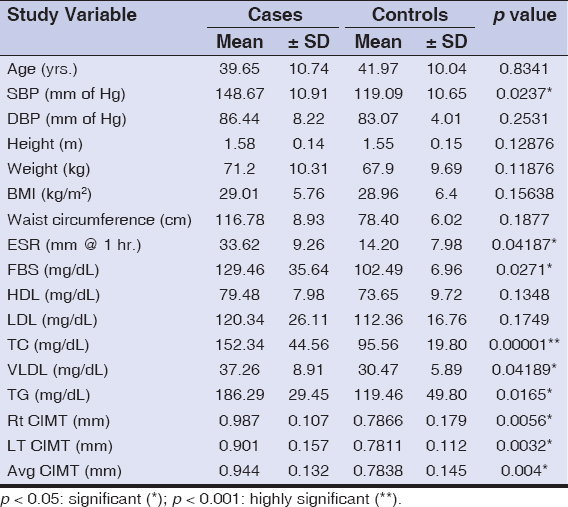 |
Table 2: Mean of study variables with respect to cases and controls |
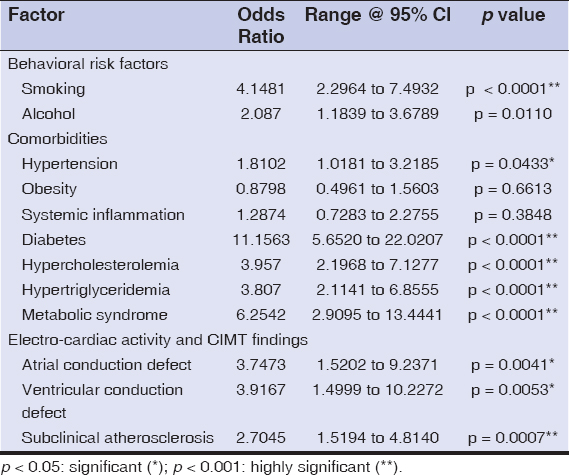 |
Table 3: Odds ratios of factors affecting psoriasis |
The mean age of the cases and controls was 39.65 ± 10.74 and 41.97 ± 10.04 years, respectively (p = 0.8341), and were all age- and sex-matched. The most common age group affected was 31 to 40 years old (36%), with a male preponderance (1.27: 1). The duration of psoriasis in our patients ranged from 8 months to 22 years (average: 14 years). The behavioral risk factors such as smoking and alcohol consumption had an association with the severe form of the disease (OR = 4.14 and 2.087, p < 0.001 and p < 0.05, respectively).
The mean PASI score was 26.19 ± 10.8, ranging from 10 to 60.8. The quality of life was moderately affected in 17% of the psoriatic patients, strongly in 15%, and extremely in 68%.
In our study, the incidence of hypertension was high in the psoriatic cases when compared with the controls (68% vs. 54%), with an OR of 1.81. The systolic blood pressure (148.67 ± 10.91 mm of Hg) was an important indicator of disease severity (p < 0.001).
The prevalence of obesity (BMI) was similar among the cases and controls (36% vs. 39%, p > 0.05, OR < 1) and was statistically insignificant. Both groups (88% vs. 83%) presented with an abnormal weight, ranging from overweight to obese (≥ 23 to ≥ 30 kg/m2).
The ESR was higher in the psoriatic patients (42%) when compared to the controls (36%), with an OR of 1.28 (p = 0.04187). Elevated fasting blood sugar levels were as high as 68%, with a significant value (p = 0.027) and a high OR (11.15) among the cases, and only 16% of the controls were affected.
Total serum cholesterol levels among the cases were higher than in the controls (69% vs. 36%): 152.34 ± 44.56 mg/dL and 95.56 ± 19.80 mg/dL, respectively (OR = 3.95). Serum triglycerides and VLDL were higher in the cases than in the controls and were statistically significant. The HDL and LDL levels were raised in the cases when compared to the controls, yet were statistically insignificant.
Metabolic syndrome was diagnosed in 41% of the patients with psoriasis and 9% in the healthy controls. This difference revealed a high statistical significance (p < 0.001) and an OR of 6.25.
A electrocardiographic study revealed an elevated P wave (22% vs. 7%, OR = 3.74) and a prolonged QTc interval (20% vs. 6%, OR = 3.91) among the cases and controls, respectively, which was statistically significant (p = 0.0041 and p = 0.0053, respectively). There were no ST-segment or T-wave variations or any other changes on the ECG.
A carotid-artery intima-medial assessment by high-resolution ultrasonography revealed an increased thickness among the cases (56%) (0.944 ± 0.132 mm) when compared to the controls (32%) (0.7838 ± 0.145 mm), which was statistically significant (p = 0.004) with an OR of 2.70. Thickened intima-media among the cases showed a statistically significant association with the severity of the disease (p < 0.001).
DISCUSSION
The severe form of psoriasis increases the risk of major adverse cardiovascular events, such as a myocardial infarction, stroke, metabolic syndrome, and peripheral vascular disease, hence termed „two plaques for one syndrome” [16].
Psoriasis is associated with an increased prevalence of cardiovascular diseases [17]. The molecular mechanisms that have been proposed include shared genetic factors, common inflammatory pathways, the secretion of adipokines, insulin resistance, lipoprotein composition and function, angiogenesis, oxidative stress, dyslipidemia, and hypercoagulability [18]. The Th1 and Th17 (IL-17, IL-6, and IL-8) cytokine pathways may mediate a vascular inflammatory cascade and the development of atherosclerosis and cardiovascular complications such as a myocardial infarction or stroke [19]. IL-17 plays both proatherogenic and atheroprotective roles [20].
In our study, the patients with severe psoriasis presented mostly in the third decade of their life and showed a male preponderance. A majority (69%) were found to have a PASI of below 30. Severe psoriasis was shown to produce a considerable effect on the quality of life, impacting daily activities and social life and causing a significant economic burden to the patients, similarly to the findings by Leon et al. and Wade et al. [21,22].
Smoking and alcohol consumption are common behavioral risk factors for psoriasis and cardiovascular diseases. In our study, these had a four-fold and two-fold association with the psoriatic patients when compared to the controls, which was comparable to the findings by Mehta et al. [10].
Obesity and being overweight characterized by excess visceral fat are thought to release proinflammatory cytokines such as IL-6 and tumor necrosis factor (TNF)-a, playing a role in both conditions [23]. In our study, both groups were equally affected, indicating an overall drift in the lifestyle and a change in food habits.
The active systemic inflammation in the body indicated by the ESR, contributed to by both psoriatic skin lesions and visceral fat due to obesity, was raised among the cases, which is similar to the findings by Lakshmi et al. [4].
Diabetes and hypercholesterolemia had a large impact on the severity of psoriasis, with a high OR, and were independent risk factors for cardiovascular diseases in our study. There was no correlation between derangements in the lipid profile and the presence of diabetes among the cases (p = 0.1726). Therefore, diabetes could not have been an underlying factor leading to derangement in the lipid profile (diabetic dyslipidemia) of the psoriatic patients in our study. Hypertriglyceridemia with elevated VLDL among the psoriasis patients was significant.
An electro-cardiac study revealed atrial (elevated P wave) and ventricular (prolonged QTc interval) conduction abnormalities among the psoriatic patients, which was similar to the findings by Bacaksiz et al. and Simsek et al. [24,25].
The highest CIMT value reported among the Indian population is 0.70 mm. CIMT values above 1.0 mm are considered clinical atherosclerosis and values between 0.7 and 1.0 are considered subclinical atherosclerosis [26,27]. In our study, the psoriatic patients were found to have subclinical atherosclerosis and the cases showed a minimal increase in thickness.
Different Indian and international studies estimating cardiovascular risk factors with psoriasis, such as hypertension, diabetes, metabolic syndrome, subclinical atherosclerosis, etc., are tabulated and compared with our study [27–30] (Table 4). Our results are supported by the majority of the published studies. The psoriatic patients with a severe form of the disease presented with high-risk factors, such as hypertension, diabetes, deranged lipid levels, metabolic syndrome, altered electrical activity, and subclinical atherosclerosis. Molecular-based targeted therapies were found to be helpful in treating both psoriasis and its cardiovascular comorbidities [31].
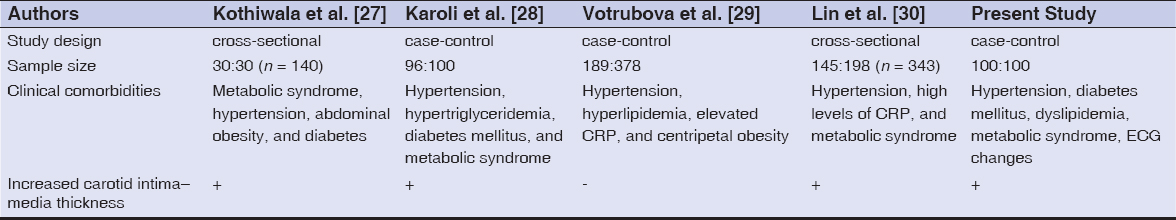 |
Table 4: Different studies estimating the risk of cardiovascular disease risk factors in psoriasis as compared to the present study |
Based on our findings, we believe that the preliminary assessment of comorbidities with routine dermatological investigations in psoriatic patients is essential to prevent possible cardiovascular mortalities. The recommended guidelines for the screening of cardiovascular comorbidities in psoriatic patients is provided by the National Psoriasis Foundation and the American Heart Association [32] (Table 5).
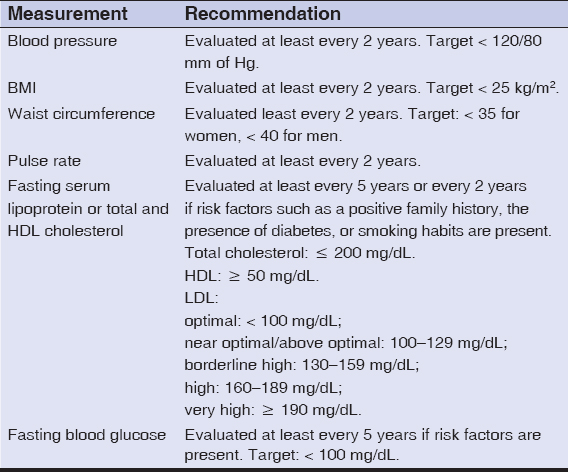 |
Table 5: American Heart Association recommendations for risk factor screening |
Limitations
The prevalence and the clinical patterns of cardiovascular disease associated with psoriasis were not assessed. Echocardiographic studies and the quantitative assessment of ECG were not possible. Studies on larger populations are required to assess the overall disease burden in the Indian population.
CONCLUSION
With the increasing prevalence and awareness of psoriasis and its association with various comorbidities, early detection and periodic screening for cardiovascular risk factors among psoriatic patients and aggressive therapeutic management are recommended to reduce adverse cardiovascular events.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Burden AD, Kirby B. Psoriasis and Related disorders. Griffiths CE, Barker J, Bleiker T et al. Rook’s Textbook of Dermatology 9th edition. Wiley Blackwell, 2016. Vol. 2;Pg 35.1-35.44.
2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis:A systematic review of incidence and prevalence. J Invest Dermatol. 201;133:377-85.
3. Sharon C, Jiquan S. Association of NLRP1 and NLRP3 gene polymorphism with psoriasis. Our Dermatol Online. 2020;11:275-83.
4. Lakshmi S, Nath AK, Udayashankar C. Metabolic syndrome in patients with psoriasis:A comparative study. Indian Dermatol Online J. 2014;5:132-7.
5. Szentpetery A, Haroon M, FitzGerald O. Cardiovascular comorbidities in psoriatic disease. Rheumatol Ther 2020;7:5-17.
6. Ganzetti G, Campanati A, Molinelli E, Offidani A. Psoriasis, nonalcoholic fatty liver disease, and cardiovascular disease:Three different diseases on a unique background. World J Cardiol. 2016;8:120-31.
7. Mehta N, Gelfand J. High density lipoprotein cholesterol function improves after successful treatment of psoriasis:a step forward in the right direction. J Invest Dermatol. 2014;134:592-5.
8. Bissonnette R, Kerdel F, Naldi L, Papp K, Galindo C, Langholff W, et al. Evaluation of risk of major adverse cardiovascular events with biologic therapy in patients with psoriasis. J Drugs Dermatol. 2017;16:1002-13.
9. Masson W, Rossi E, Galimberti ML, Krauss J, Navarro Estrada J, Galimberti R, et al. Mortality in patients with psoriasis. A retrospective cohort study. Med Clin (Barc). 2017;148:483-8.
10. Alsamarai AM, Alobaidi AHA. Psoriasis is a systemic disease:A proposed approach for inflammation scale calculation. Our Dermatol Online. 2021;12:381-6.
11. Siegel D, Devaraj S, Mitra A, Raychaudhuri SP, Raychaudhuri SK, Jialal I. Inflammation, atherosclerosis, and psoriasis. Clin Rev Allergy Immunol. 2013;44:194-204.
12. Augustin M, Vietri J, Tian H, Gilloteau I. Incremental burden of cardiovascular comorbidity and psoriatic arthritis among adults with moderate-to-severe psoriasis in five European countries. J Eur Acad Dermatol Venereol. 2017;31:1316-23.
13. Feldman SR, Tian H, Gilloteau I, Mollon P, Shu M. Economic burden of comorbidities in psoriasis patients in the United States:Results from a retrospective U.S. database. BMC Health Serv Res. 2017;17:337.
14. Strober B, Ryan C, van de Kerkhof P, van der Walt J, Kimball AB, Barker J, et al. International Psoriasis Council Board Members and Councilors. Recategorization of psoriasis severity:Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82:117-22.
15. Sof K, Aouali S, Bensalem S, Zizi N, Dikhaye S. Dermatoses in the hospital and their impact on quality of life. Our Dermatol Online. 2021;12:462-3.
16. Bukhari I, Ismail M, Hasan M, Alzahrani A. Perspectives in psoriasis, psoriatic arthritis, non-alcoholic fatty liver disease and atherosclerosis in psoriasis. Our Dermatol Online. 2018;9:447-52.
17. Coumbe AG, Pritzker MR, Duprez DA. Cardiovascular risk and psoriasis:Beyond the traditional risk factors. Am J Med. 2014;127:12-8.
18. Hu SC, Lan CE. Psoriasis and Cardiovascular Comorbidities:Focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
19. Furuhashi T, Saito C, Torii K, Nishida E, Yamazaki S, Morita A. Photo(chemo)therapy reduces circulating Th17 cells and restores circulating regulatory T cells in psoriasis. PLoS One. 2013;8:e54895.
20. Owczarczyk-Saczonek A, Placek W. Interleukin-17 as a factor linking the pathogenesis of psoriasis with metabolic disorders. Int J Dermatol. 2017;56:260-8.
21. Leon A, Beroukhim K, Danesh M, Nguyen C, Lee K, Farahnik B, et al. The correlation between the Dermatology Life Quality Index and the Psoriasis Area Severity Index. J Psoriasis Psoriatic Arthritis. 2015;1:26-30.
22. Wade AG, Crawford GM, Young D, Leman J, Pumford N. Severity and management of psoriasis within primary care. BMC Fam Pract. 2016;17:145.
23. Wheatley R, Brooks J, Stumpf B, Boh E. Obesity, diet, and inflammation in psoriasis. J Psor Psor Arthritis. 2017;2:97-101.
24. Bacaksiz A, Erdogan E, Tasal A, Vatankulu MA, Kul S, Sevgili E, et al. Electrocardiographic P-wave characteristics in patients with psoriasis vulgaris. Ups J Med Sci. 2013;118:35-41.
25. Simsek H, Sahin M, Akyol A, Akdag S, Ozkol HU, Gumrukcuoglu HA, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. Scien World J. 2013:9:012-5.
26. Kasliwal RR, Bansal M, Desai D, Sharma M. Carotid intima-media thickness:Current evidence, practices, and Indian experience. Indian J Endocr Metab. 2014;18:13-22.
27. Kothiwala SK, Khanna N, Tandon N, Naik N, Sharma VK, Sharma S, et al. Prevalence of metabolic syndrome and cardiovascular changes in patients with chronic plaque psoriasis and their correlation with disease severity:A hospital-based cross-sectional study. Indian J Dermatol Venereol Leprol. 2016;82:510-8.
28. Karoli R, Fatima J, Shukla V, Dhillon KS, Khanduri S, Maini S, et al. A study of cardio metabolic risk profile in patients with psoriasis. JAssoc Physicians India. 2013;61:798 803.
29. Votrubova J, Juzlova K, Dzambova M, Hercogova J, Gopfertova D. Cardiovascular comorbidities in patients with psoriasis:Risk profile including carotide ultrasonography assessed in hospital-based case control study. Acta Dermatovenerol Croat. 2016;24:187-92.
30. Lin YC, Dalal D, Churton S, Brennan DM, Korman NJ, Kim ES, et al. Relationship between metabolic syndrome and carotid intima-media thickness:Cross-sectional comparison between psoriasis and psoriatic arthritis. Arthritis Care Res (Hoboken). 2014;66:97-103.
31. Curtis JR, Danila MI, Chen L, Chan B, Ehst B, Xie F, et al. Risk of Cardiovascular outcomes among psoriasis patients treated with biologics and other systemic agents. J Psoriasis Psoriatic Arthritis. 2016;1:128-37.
32. Cai J, Cui L, Wang Y, Li Y, Zhang X, Shi Y. Cardiometabolic comorbidities in patients with psoriasis:Focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-6410-3194 http://orcid.org/0000-0002-6410-3194 |



Comments are closed.