Metastasis of a widespread malignant melanoma in the bladder and the upper urinary tract: A case report
Adil Mellouki 1,2, Jauffray Oliva1, Ouima Justin Dieudonné Ziba2, Laurent Daniel3, Eric Lechevallier1, Michael Baboudjian1
1,2, Jauffray Oliva1, Ouima Justin Dieudonné Ziba2, Laurent Daniel3, Eric Lechevallier1, Michael Baboudjian1
1Department of Urology, Conception Academic Hospital, Marseille, France, 2Department of Urology, Teaching Hospital Hassan II, Fès, Morocco, 3Department of Pathological Anatomy and Cytology, Conception Academic Hospital, Marseille, France
Corresponding author: Adil Mellouki, MD
How to cite this article: Mellouki A, Oliva J, Dieudonné Ziba OI, Daniel L, Lechevallier E, Baboudjian M. Metastasis of a widespread malignant melanoma in the bladder and the upper urinary tract: A case report. Our Dermatol Online. 2022;13(2):155-157.
Submission: 02.12.2021; Acceptance: 13.02.2022
DOI: 10.7241/ourd.20222.8
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
The urinary tract is an uncommon site of metastatic melanoma. Herein, we discuss a rare case of melanoma with metastatic involvement of the bladder and upper urinary tract and review its surgical management based on prognosis. We report the case of a 38-year-old male with biopsy-proven ureteral and bladder metastasis of cutaneous melanoma. The management of these metastases is challenging. Endoscopic treatments allowed to control local symptoms without associated serious adverse events. Radical surgical operations such as nephroureterectomy could be envisaged for completely resectable tumors in a patient responding well to systemic therapies.
Key words: Melanoma; Metastasis; Bladder; Upper urinary tract
INTRODUCTION
Cutaneous melanoma has the potential to spread to distant organs, causing more than 60,000 deaths annually worldwide [1]. The most common clinically apparent sites of distant metastases in melanoma patients are the skin, lungs, brain, liver, bones, and intestines [2–4]. Herein, we discuss a rare case of melanoma with metastatic involvement of the bladder and upper urinary tract and review its surgical management based on prognosis.
CASE REPORT
A 38-year-old male with multi-organ metastatic melanoma was referred to our department for gross hematuria and right renal colic. A CT scan revealed a large, circumferential tumor of the right ureteropelvic junction and right lumbar ureter (Fig. 1). Flexible cystoscopy was then performed, which found a small synchronous bladder localization.
 |
Figure 1: CT scan: The dotted line shows the circumferential tumor of the ureteropelvic junction. The asterisk corresponds to the dilatation of the upper urinary tract prior to the tumor. |
The patient subsequently underwent a transurethral resection to remove the tumor in the bladder and right ureteroscopy with a biopsy of the tumor in the upper urinary tract. Each sample was sent to an experienced pathologist. Retrograde ureteral stenting was employed due to the dilatation of the upper urinary tract.
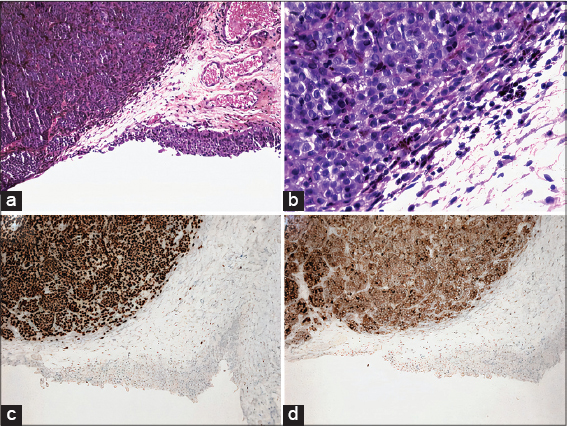
Pathological findings confirmed metastasis of melanoma in the urinary tract. Fig. 3 shows bladder resection specimens with an ulcerative lesion and tumor sheets next to a dystrophic urothelium (Fig. 2a). Some tumor cells were highly pigmented (Fig. 2b). Immunohistochemistry confirmed melanoma cells with SOX10 antibody (Fig. 2c) and positive BRAF V600E staining (Fig. 2d).
Due to the unresectability of multiple secondary lesions, no intention-to-treat surgery was considered for the tumor in the upper urinary tract. The patient was a poor responder to immunotherapy and had a disease progression. However, the patient was treated with conservative management by resection and coagulation with holmium laser, and neither hematuria nor renal colic was reported after three months of follow-up.
DISCUSSION
Historically, systemic therapy for metastatic melanoma was associated with poor response rates, yet the last decade has seen the development of multiple targeted and immune therapies that have shown a survival benefit. The surgical management of the metastatic site could be envisaged for completely resectable tumors. These stage IV patients exhibit improved survival, regardless of the site and the number of metastases [5,6].
The genitourinary tract is an uncommon site of metastatic melanoma. The treatment of genitourinary metastases is challenging and relies primarily on prognosis. Metastasectomy should be considered for local control in oligometastatic disease if a complete macroscopic resection may be obtained while any remaining distant disease is stable on immunotherapy. Transurethral resection of tumors in the bladder and nephroureterectomy provide the best local control of lower and upper urinary tract involvement, respectively [7]. In patients with a poor response to immunotherapy, palliative nephroureterectomy should be considered with caution. However, retrograde ureteral stenting and transurethral resection allowed local control of symptoms, in particular, gross hematuria, which may interrupt the administration of necessary systemic therapies and lead to more hospital admissions [7].
CONCLUSION
The treatment of metastatic melanoma is challenging. Although the genitourinary tract is a rare site of metastasis, early detection is important as effective therapies are available, improving control of local symptoms and the potential disease progression.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016:A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553-68.
2. Damsky WE, Rosenbaum LE, Bosenberg M. Decoding melanoma metastasis. Cancers (Basel). 2010;3:126-63.
3. Limam SAM, Erebih CE, Beyrouk A, Boye KI, Didi EH, Ely SO, et al. [Acral melanoma of the foot:A study of 9 cases and guidelines update]. Our Dermatol Online. 2019;10:23-9.
4. Saàdani CH, Gallouj S, Zinoune S, Senhaji G, Baybay H, Mernissi FZ, et al. Diffuse melanosis cutis secondary to metastatic malignant melanoma:Case report. Our Dermatol Online. 2019;10:79-81.
5. Howard JH, Thompson JF, Mozzillo N, Nieweg OE, Hoekstra HJ, Roses DF, et al. Metastasectomy for distant metastatic melanoma:Analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I). Ann Surg Oncol. 2012;19:2547-55.
6. Bebe FN, Hu S, Brown TL, Tulp OL. Metastatic melanoma in Florida, 1996-2010:Racial, demographic, occupational and tumor characteristics, and burden of metastasis. Our Dermatol Online. 2018;9:369-79.
7. Nair BC, Williams NC, Cui C, Summers D, Mastrangelo MJ, Hubosky SG, et al. Conjunctival melanoma:Bladder and upper urinary tract metastases. J Clin Oncol. 2011;29:e216-9.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.