COVID-19 and pemphigus: A descriptive series of twelve cases
Zoubida Mehsas , Soukaina Sektaoui, Meriem Meziane, Nadia Ismaili, Leila Benzekri, Karima Senouci
, Soukaina Sektaoui, Meriem Meziane, Nadia Ismaili, Leila Benzekri, Karima Senouci
Department of Dermatology and Venereology, CHU Ibn Sina, Mohamed V University of Rabat, Morocco
Corresponding author: Zoubida Mehsas, MD
How to cite this article: Mehsas Z, Sektaoui S, Meziane M, Ismaili N, Benzekri L, Senouci K. COVID-19 and pemphigus: A descriptive series of twelve cases. Our Dermatol Online. 2022;13(2):148-151.
Submission: 27.12.2021; Acceptance: 07.02.2022
DOI: 10.7241/ourd.20222.6
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Patients with pemphigus receiving immunosuppressive therapy are considered at risk of developing severe forms of COVID-19. We aimed to analyze the clinical and evolutionary characteristics of COVID-19 infection in patients with pemphigus receiving general corticosteroid therapy by a retrospective descriptive study of twelve cases.
Patients and Methods: We identified´ pemphigus cases that developed a COVID-19 infection. The data collected included the characteristics and treatment of pemphigus and the features and course of COVID-19 infection.
Results: Twelve patients were followed at our department for pemphigus and a developed COVID-19 infection. Nine patients had a severe form of pemphigus, and three were in clinical remission. The infection was severe in three patients. The evolution was good in all patients.
Discussion: Our study suggests that systemic corticosteroid therapy in patients with severe forms of pemphigus may be a protective factor against severe forms of SARS-CoV-2 infection. This theory should be evaluated by a larger study.
Key words: COVID-19; Corticosteroid Therapy; Pemphigus; Rabat; Morocco
INTRODUCTION
COVID-19, a disease resulting from infection by the virus SARS-CoV-2, was first described in late December 2019, in Wuhan, China. On March 11, 2020, the World Health Organization (WHO) declared the disease a pandemic responsible for more than one million deaths worldwide [1].
The severity of coronavirus disease-2019 (COVID-19) is related to age, the male sex, and the presence of comorbidities: obesity, pulmonary and cardiovascular pathologies, diabetes, and cancers [2].
For nearly a year, numerous studies have been published to provide a better understanding of the virus, its management, and its consequences, including in patients with pemphigus. Indeed, patients suffering from pemphigus, especially its severe form, and receiving immunosuppressive treatment are considered at risk of developing severe forms of COVID-19.
We evaluated the risk of COVID-19 and its severe forms in these patients by a retrospective, descriptive study of twelve cases of pemphigus followed at our department.
Our objective was to analyze the clinical and evolutionary characteristics of COVID-19 infection in patients with pemphigus receiving immunosuppressive therapy.
MATERIALS AND METHODS
This was a retrospective, descriptive study including all cases of pemphigus followed at the dermatology department of Ibn Sina Rabat Hospital and having developed a COVID-19 infection confirmed by a positive SARS-CoV-2 PCR test over a period of eleven months (between March 2020 and February 2021).
RESULTS
Twelve patients followed at the dermatology department of Ibn Sina Rabat Hospital for pemphigus (six cases of superficial pemphigus, six cases of deep pemphigus, one of which was vegetative) developed a COVID-19 infection between March 2020 and February 2021.
Table 1 details the clinical characteristics of these twelve patients.
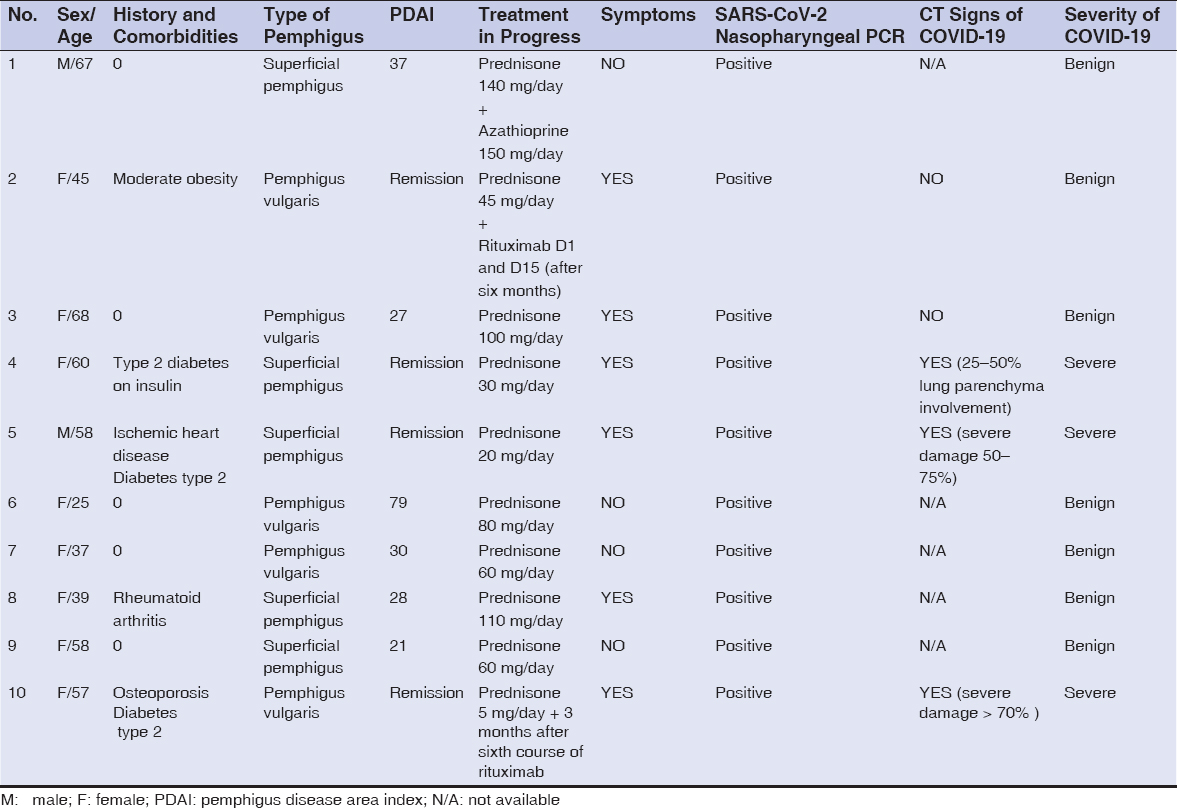 |
Table 1: Characteristics of the patients with pemphigus and COVID-19 infection. |
There were ten females and two males, with a sex ratio of 0.2. The mean age of the patients on diagnosis was 50.9 years (25 to 68 years).
Four patients were diabetic, three were moderately to severely obese, and one had ischemic heart disease under treatment. One patient was hypertensive and another was followed for rheumatoid arthritis.
Nine had a severe form of pemphigus, with a PDAI between 21 and 98. These nine patients had been receiving a high dose of general corticosteroid therapy between 60 and 140 mg/day. Two were on 150 mg of azathioprine in combination. One patient had received rituximab D1 D15 (2 g).
Three cases were in clinical remission under low-dose corticosteroid therapy (5 to 20 mg/day), among which one received its M6 dose of rituximab (1 g) three months previously.
Nine of the twelve cases were in contact with suspected or confirmed cases of COVID-19, while no exposure to risk was noted for the remaining three cases.
A SARS-CoV-2 PCR test was positive by a nasopharyngeal swab in all twelve patients. Regarding the clinical features of COVID-19 infection:
The infection was considered severe in three patients. These patients presented hypoxia, dyspnea, and a CT scan > 50% with a biological inflammatory syndrome. These three patients were hospitalized in an intensive care unit and received oxygen therapy, curative anticoagulation, and antibiotic therapy.
Among these 3 patients, one had a significant inflammatory syndrome and received tocilizumab (Note that this patient had comorbidities such as diabetes and severe obesity. This patient was in complete remission of her pemphigus and was, therefore, only receiving 5 mg of prednisone and received an M6 dose of rituximab three months previously.)
Four of the twelve cases were symptomatic with mild symptoms of flu-like illness and anosmia. Five were asymptomatic.
No systemic treatment for pemphigus was withheld in these twelve patients. All patients had a favorable outcome and no deaths were reported.
Moreover, we noted no clinical cutaneous aggravation of pemphigus in our patients.
DISCUSSION
This was a retrospective, descriptive study of twelve patients followed for pemphigus, who developed a COVID-19 infection during the previous eleven months. Among these twelve patients, nine had a severe form of pemphigus and had been receiving a high dose of systemic corticosteroid therapy (between 60 and 140 mg/day) and had developed a mild form of SARS-CoV-2 infection. The three of the twelve patients who had a severe COVID-19 infection were on low doses of corticosteroid therapy (between 5 and 20 mg/day), yet had mainly comorbidities such as diabetes and severe obesity. This suggests that high-dose systemic corticosteroid therapy had had a protective role and had improved the prognosis of COVID-19 infection in our series.
The literature has not yet reported that patients with autoimmune bullous disease have a higher risk of being infected with SARS-CoV-2 compared to healthy individuals. However, this seems possible due to the immunosuppressive context of the autoimmune disease itself and its treatment [3].
There is a limited number of series studying the course of COVID-19 in patients with pemphigus. In an Italian series including 31 patients with pemphigus, seven patients (one male, six female; mean age: 68.3 ± 9.7) presented with symptoms suspected of COVID-19, six patients (19.4%) with mild to moderate symptoms, and one (3.2%) with severe symptoms requiring hospitalization
In contrast to our series, all patients were in remission and had, therefore, been on low-dose systemic corticosteroid therapy with a mean dose of 5.9 ± 6.2 mg/day. This ongoing treatment was only interrupted for the hospitalized patient [4].
In an Italian series including nine patients with pemphigus, only one patient tested positive for SARS-CoV-2. The patient was a 65-year-old female with pemphigus lasting for more than three years and on mycophenolate mofetil for 38 months. The patient presented with severe nausea, fever, anorexia, asthenia, and the next day tested positive for SARS-CoV-2. She stopped treatment two days after the diagnosis of COVID-19. She gradually improved and was completely free of symptoms twelve days after diagnosis [5,6].
In an Iranian study, a total of 45 pemphigus patients who underwent rituximab treatment between 2014 and 2020 were included. The authors identified five cases (four female, one male; mean age: 41.8 ± 9.6 years) of confirmed COVID-19. Among these five patients, four had mild symptoms related to COVID-19. Only one incidentally diagnosed patient was asymptomatic All patients were on prednisolone therapy at a dose of 5–10 mg/day. Two patients had additionally received pulsed therapy with methylprednisolone and azathioprine. None received rituximab during the year preceding the pandemic [6].
Despite concerns that corticosteroids induce the inhibition of antiviral immunity and, thus, may disrupt the viral load, low-dose systemic corticosteroids appear to play a role in treating severe COVID-19 infections [7]. Our study confirms this hypothesis and suggests that high-dose systemic corticosteroids had a protective role and improved the outcome of severe COVID-19.
In their expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic, Kasperkiewicz et al. suggested that immunomodulatory therapies, including systemic corticosteroids, should be continued if necessary, as unwarranted withdrawal may cause disease exacerbation associated with high morbidity and mortality [8].
For rituximab, Shakshouk et al. advocated temporarily deferring rituximab therapy to delay the peak of immunosuppression in patients during the peak incidence of COVID-19 [6].
They also suggested that topical corticosteroids and prednis(ol)one ≤ 10 mg/day may be continued in patients infected with SARS-CoV-2, while prednis(ol)one > 10 mg/day may be reduced, taking into account the disease activity and severity, comorbidities, age, and severity of COVID-19, and therefore a withdrawal or significant reduction in the dose of systemic corticosteroids should be avoided, especially in patients with severe forms of autoimmune bullous diseases [9].
Moreover, the hospital management of severe and critical forms of COVID-19 relies mainly on two possibly associated strategies: decreasing viral replication and preventing an exacerbated inflammatory response. Corticosteroids are the only treatment at present to have demonstrated a benefit in reducing the risk of mortality in oxygen-dependent patients with a critical form of COVID-19 [10]. However, several prospective trials have been conducted to evaluate the efficacy of corticosteroid therapy in the management of severe forms of COVID-19. The RECOVERY Trial randomized the use of dexamethasone (6 mg/day for ten days) in addition to standard therapy in patients hospitalized with COVID-19 infection. In patients treated with corticosteroids (2104 vs. 4321), there was a decrease in 28-day mortality (the primary objective of the trial) for ventilated or oxygen-requiring patients, and a decrease in the risk of mechanical ventilation for patients on oxygen. This effect was absent in patients not requiring oxygen. A possible deleterious effect has been suggested in this population [10].
In fact, the WHO and several learned societies do not contraindicate the use of corticosteroids in cases in which their benefits are well established and recommend that they be continued as background treatment [11].
CONCLUSION
Our study has certain limitations. The analysis of the records was retrospective and not all data was always known. The number of patients was low making it difficult to interpret some data.
Despite these biases, the study provides insight into some aspects of COVID-19 in patients with severe pemphigus receiving high doses of systemic corticosteroid therapy and suggests the impact of this treatment on the successful outcome of SARS-CoV-2 infection despite the presence of comorbidities.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Abdollahimajd F, Shahidi-Dadras M, M Robati R, Dadkhahfar S. Management of pemphigus in COVID-19 pandemic era:A review article. Arch Acad Emerg Med. 2020;8:e51.
2. Carugno A, Sena P, Raponi F, Robustelli Test E, Vezzoli P. Patients with bullous skin disease in a high-epidemic COVID-19 area, Bergamo, Italy. Br J Dermatol. 2020;183:589-91.
3. Balestri R, Rech G, Girardelli CR. Occurrence of SARS-CoV-2 during mycophenolate mofetil treatment for pemphigus. J Eur Acad Dermatol Venereol. 2020;34:e435-6.
4. Nobari NN, Goodarzi A. Patients with specific skin disorders who are affected by COVID-19:What do experiences say about management strategies?A systematic review. Dermatol Ther. 2020;e1386.
5. Shahidi-Dadras M, Abdollahimajd F, Ohadi L. COVID-19 in pemphigus vulgaris patients with previous rituximab therapy:A tele-medicine experience. J Dermatol Treat. 2020;1-2.
6. Shakshouk H, Daneshpazhooh M, Murrell DF, Lehman JS. Treatment considerations for patients with pemphigus during the COVID-19 pandemic. J Am Acad Dermatol. 2020;82:e235–6.
7. Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al. Systemic corticosteroids for the treatment of COVID-19. Database Syst Rev. 2021;8:CD014963.
8. Kasperkiewicz M, Schmidt E, Fairley JA. Expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2020;34:e302-3.
9. Horby P, Mafham M, Linsell L, Bell J.L, Staplin N. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-40.
10. Cui Y, Sun Y, Sun J, Liang H, Ding X, Sun X, Wang D, Sun T, et al. Efficacy and safety of corticosteroid use in coronavirus disease 2019 (COVID-19):A systematic review and meta-analysis. Infect Dis Ther. 2021;10:2447-63.
11. Herman P, Vincent c, Parietti Winkler C, Loundon N, Couloigner V, Tankere F, et al. Conseils de bonne pratique. Corticothérapie en ORL en contexte de pandémie COVID-19. Ann FrançD’Oto-Rhino-Laryngol Pathol Cervico-Faciale. 2020;137:292-4.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.