Cutaneous lupus: Immunofluorescence and lupus band test
Hassiel Aurelio Ramírez-Marín1, Ahmad Aleisa2, Anabell Andrea Lima-Galindo3, Silvia Mendez-Flores 3
3
1Department of Dermatology, Weill Cornell Medical College, New York, NY, USA, 2Department of Pathology, Medical University of South Carolina, Charleston, USA, 3Department of Dermatology, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” Mexico City, México
Corresponding author: Silvia Mendez-Flores, MD, MSc., PhD.
How to cite this article: Ramírez-Marín HA, Aleisa A, Lima-Galindo AA, Mendez-Flores S. Cutaneous lupus: Immunofluorescence and lupus band test. Our Dermatol Online. 2022;13(2):202-209.
Submission: 28.12.2021; Acceptance: 14.02.2021
DOI: 10.7241/ourd.20222.21
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Cutaneous lupus erythematosus is an autoimmune disease with a broad range of clinical findings. There are several methods to conduct a diagnostic approach, among these stands direct immunofluorescence, in which we can find the Lupus Band Test (LBT) consisting of a band of granular deposits of immunoglobulins and complement along the dermo-epidermal junction. It can have a high sensitivity and specificity for diagnosis in some cases, according to the biopsy site, the constituents of immunorreactants found at the dermoepidermal junction, and the morphology and intensity of the immunofluorescent band. Certain limitations of the test should be considered when interpreting the results. Although useful in diagnosis, the lupus band is not considered a pathognomonic sign of erythematous lupus. About one-third of patients with positive direct immunofluorescence on a skin biopsy do not have systemic lupus erythematosus (SLE). A positive LBT in non-lesional photoprotected skin represents a criterion with high specificity to identify patients with SLE.
Key words: Direct Immunofluorescence; Lupus Erythematosus; Systemic; Lupus Erythematosus; Cutaneous; Lupus Erythematosus; Discoid; Immunoglobulins
ABBREVIATIONS
- DI: Direct immunofluorescence
- IA: Immunoreactive agents
- LE: Lupus erythematosus
- CLE: Cutaneous lupus erythematosus
- SCLE: Subacute cutaneous lupus erythematosus
- DLE: Discoid lupus erythematosus
- SLE: Systemic lupus erythematosus
- NLE: Neonatal lupus erythematosus
- LBT: Lupus band test
- DEJ: Dermoepidermal junction
- DNA: deoxyribonucleic acid
- C5b-9: Complement complex 5b-9
- ESR: Erythrocyte sedimentation rate
- NPV: Negative predictive value
- PPV: Positive predictive value
- ESJ: Epithelial-stromal junction
- ANA: Antinuclear antibodies:
- LET: Lupus erythematosus Tumidus
INTRODUCTION
Lupus erythematosus (LE) is an autoimmune disease associated with multisystemic inflammation. There are four main types: neonatal lupus erythematosus (NLE), cutaneous lupus erythematosus (CLE), drug-induced lupus and systemic lupus erythematosus (SLE) [1]; three types of CLE are recognized: acute, subacute and chronic [2]. All of these subtypes share clinical, histological, and immunological characteristics. Circulating autoantibodies of LE are detected with serological tests and cutaneous immune deposits by direct immunofluorescence (DI), which can be useful for making the diagnosis when there is clinical and/or histopathological suspicion [3,4]. Evaluation by direct immunofluorescence of lesional or non-lesional skin to search for a diffuse band of granular deposits made out of immunoreactive agents (IA) along the dermoepidermal junction (DEJ) as part of the cutaneous lupus (CL) and systemic lupus erythematosus (SLE) diagnostic approach is historically recognized as the “lupus band test” (LBT). The range of DI positivity rates is very wide, ranging from 39% for subacute cutaneous lupus erythematosus (SCLE), 55-70% for discoid lupus erythematosus (DLE), 80% for acute cutaneous lupus erythematosus (ACLE), and from 50% to 90% for SLE [5]; therefore its diagnostic value is not very clear for the diagnosis of LE. On the other hand, it has been considered that DI could be useful in distinguishing between the different subtypes of CLE since the frequency of deposits, their morphology and position vary between the different subtypes, in addition, it can be helpful to differentiate CLE from other dermatoses that may have similar clinical findings (such as rosacea, facial telangiectasias, lymphocytoma cutis, pemphigus erythematosus, lichen planopilaris (Fig. 1), pseudopelade of Brocq, drug-induced LE, pharmacodermias and cutaneous vasculitis) or overlapping histopathological findings, as in polymorphous light eruption or benign lymphocytic infiltration of the skin [2,3].
The correlation between clinical, histopathology, immunofluorescence, and serologic profiles remains crucial for accurate diagnosis of cutaneous lupus erythematosus [3]. New diagnostic methods such as ex vivo confocal laser scanning microscopy have tried to identify histopathological features and perform the lupus band test at the same time, albeit with a lower performance than conventional DI [4], making conventional DI microscopy a still one of the most accurate diagnostic tools for cutaneous inflammatory lesions.
Direct Immunofluorescence in Lupus
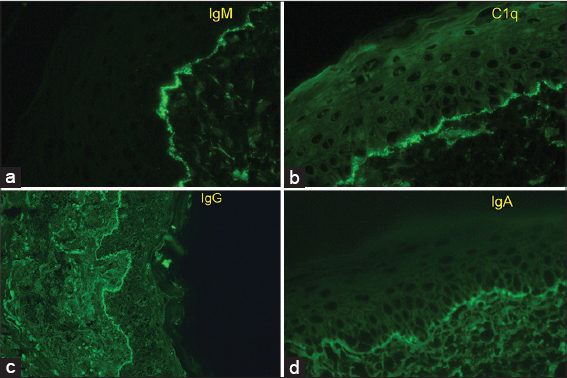
Throughout the DEJ in DI of patients with any type of lupus, the most frequent immune deposits are IgG and IgM (Fig. 2) [3]. Immunoreactants in the DEJ are believed not to be antibodies to the basement membrane, but to represent circulating immune complexes of DNA and antinuclear antibodies trapped in the DEJ [6]; UV-damaged keratinocyte DNA is released and can diffuse through the basement membrane to bind to type IV collagen and serve as antigen for circulating antinuclear antibodies [7].
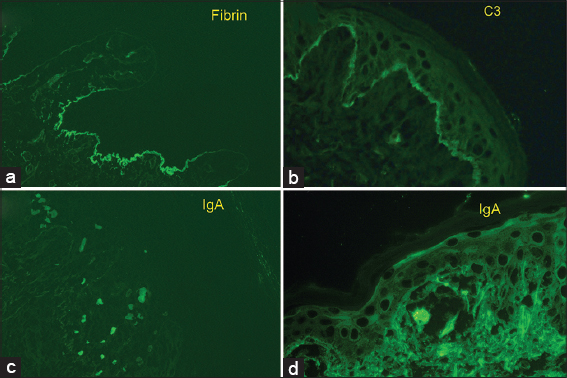 |
Figure 2: Granular basement membrane zone deposition of IgG (1+), IgA (trace, (C3(trace), C1q (3+) Kappa (1+), and lambda (1 to 2+) compatible with Lupus Erythematosus. |
Several fluorescence patterns have been defined, the most described being: linear and granular, which can be seen in low-power microscopy as thin or thick bands [5]. Within the granular pattern, which is the most frequent6, when viewed with a high-powered microscope, such as confocal laser scanning microscopy other patterns can be identified: homogeneous, fibrillar, dotted, shaggy, lumpy, linear, and filiform [7], classification not widely used in clinical practice due to the lack of availability of this type of microscope. All of these usually present continuously, an interrupted form is less specific and can be seen in other dermatoses. In addition to these immune deposits, cytoid bodies can also be seen in the DEJ, which represent basal keratinocytes that “fell” to the papillary dermis and adhered to immunoglobulins (most often IgM and IgA) and circulating complement [3].
Systemic immunosuppressive treatment is associated with a lower frequency of positive DI. In addition, while fluorescence intensity is related to DNA antibody titers [4], it does not correlate with the degree of inflammation in SLE lesions [5]. There is also a variation in the positivity of the lesion skin test with a cephalocaudal gradient, head lesions are more frequently positive than those of the trunk [5,7]. Unlike histopathology, DI does not reflect real time changes but displays any past insult on the structures involved [3].
The particular characteristics of DI for different types of lupus are discussed below.
Discoid Lupus Erythematosus
The possibility of finding immune deposits in DLE depends on the biopsy site, previous treatments and duration of the lesion; 60-94% of lesional skin biopsies are reported to have immune deposits. In one study, DI was positive in 96% of biopsies of facial lesions, while it was positive in only 65% of lesions in other photoexposed regions and 30% in non-photo-exposed areas [8]. The frequency of positive DI in lesions under treatment (for more than three weeks before biopsy) is lower than that found in untreated lesions, and finally, it has been seen that while the duration of the lesion increases, The frequency of positive DI also does so by up to 82% in lesions older than one year, while it has been found that only a third of lesions less than 1 month long have a positive DI [8]. To confirm the diagnosis of DLE, the most appropriate site for biopsy is the oldest, untreated lesion, preferably from an area not usually exposed to the sun [9,10].
In the immune deposits of the DEJ there is predominance of IgG (77-80%) [11,12] and intense granules of C5b-9 (60%); Both are typically absent in endothelium or keratinocytes [10]. Although these immune deposits can be seen in many other diseases (including rosacea, lichen planus, and primary biliary cirrhosis), the immunoglobulin class may be helpful in distinguishing DLE, as IgG deposits are more specific to DLE [3]
Subacute Cutaneous Lupus Erythematosus
The positivity of DI varies from 54% to 100% in the lesions of SCLE, from 18% to 100% in non-lesional skin and from 0-100% in non-lesional and non-photoexposed skin [11,12].
Unlike DLE, immune deposits are also found in basal keratinocytes and this has been reported as a more specific pattern for SCLE, consisting of granular fluorescence for IgG and C5b-9 in the cytoplasm and nucleus of basal keratinocytes [3,10], this is probably an ANA in situ phenomenon, thought to be a reflection of the binding of anti-Ro (SS-A) or anti-La (SS-B) antibodies or both to their respective keratinocyte antigen [12,13]. This pattern has been shown to be an independent factor from serological findings in patients with and without anti-Ro antibodies [10] and has also been reported in patients with anti-Ro antibodies (SS-A) who do not have SCLE [3]. The deposition of C5b-9 along the DEJ is observed in 66% of cases [10] and vascular deposits are generally absent, except in patients with drug-associated SCLE [10,13].
Systemic Lupus Erythematosus
The prevalence of immunoglobulins and complement deposition in SLE, as in DLE, depends on several factors. In SLE, immune deposits can be present in 4 sites [14,15]: in the DEJ, which is the most characteristic site, in the cytoid bodies (which represent basal keratinocytes that have suffered necrosis), in the superficial dermis vessels walls (similar to vasculitis) and finally, in the keratinocytes nuclei, a much less frequent finding reported for the first time in patients with mixed connective tissue disease and usually seen in patients with antibodies against U1RNP, although it can be seen in patients with other antinuclear antibodies [15].
85% of patients with SLE have multiple immune deposits throughout the DEJ, the most frequently found being IgG, IgM, IgA, C1q and C3 and generally in combination; About 45% of patients demonstrate IgG and IgM with or without C3 [16]. The presence of IgG and C5b-9 deposits in the DEJ is observed in patients with SLE with anti-Ro/SSA antibodies, patients in whom there is circulating lupus anticoagulant or vasculitis demonstrated in the biopsy [14]. Intense deposition of C5b-9 granules has been observed in 80% of lesional skin [10]. Other immune deposits include IgD, IgE, and fibrin. In the cell nuclei the main immunoglobulin found is IgG (in situ ANA phenomenon) [15–17].
Nuclear and cytoplasmic granular deposits of IgG and C5b-9 have been observed in keratinocytes and blood vessels when patients present with extractable nucleus antigen (ENA) antibodies: Ro, La, Sm, or U1RN; while deposits are weak or absent when these antibodies are not present [14].
The lupus band dotted pattern, consisting of multiple small round points of fluorescence, is the most commonly type seen in clinically normal skin of SLE, and has been associated with disease of less than one year of evolution; while a filiform pattern has been associated with an evolution of more than one year [14]. In chronic atrophic, hypertrophic, hyperkeratotic, or scaly skin lesions, a well-defined homogeneous or “solid” band of bright fluorescence has been reported, while in acute erythematous and edematous lesions a fibrillar pattern consisting of short, stacked and bright fibrils has been seen [7]. The intensity of the DEJ fluorescence is related to the levels of antibodies against double-stranded DNA and therefore to disease activity [3].
Serological tests are more reliable than DI in the diagnosis of SLE, since the presence of high titres of antinuclear antibodies by immunofluorescence and antibodies specific to several nuclear antigens such as U1RNP and Sm are very characteristic of SLE [3].
Lupus band test in non-lesional or non-photoexposed skin in SLE
In SLE there may be alterations in DI of healthy skin, this could be useful for early diagnosis [7] and in the differential diagnosis with DLE or CLE, since skin lesions may be identical and the DI characteristics of the affected skin are similar in all these entities, but in the non-lesional skin DI of patients with DLE or SCLE there are no alterations [18]. However, the frequency of positive DI in patients with DLE is lower in healthy skin, and varies between photoexposed and non-photoexposed skin (Table 1), while in injured skin it is positive in 50-100% of the time [3].
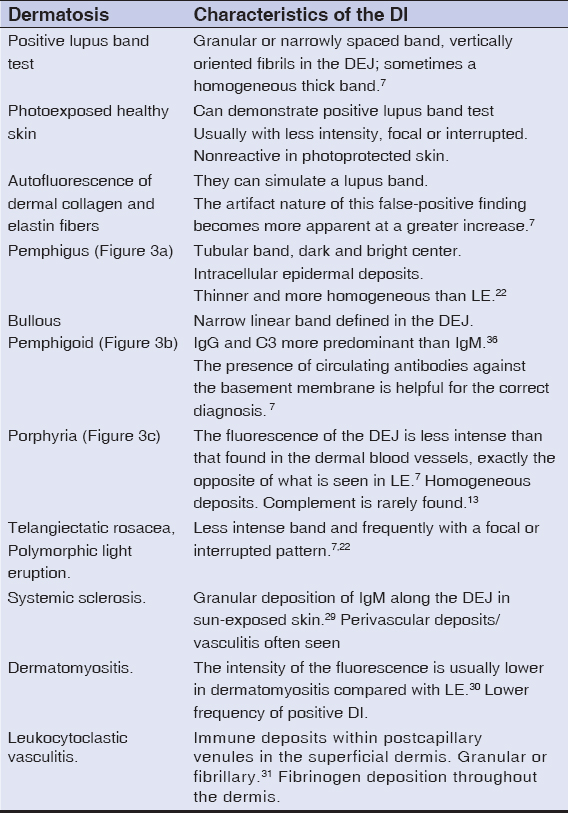 |
Table 1: Differential diagnoses for lupus band in other dermatoses. |
The lupus band test in patients with SLE is positive in 70%-80% of photoexposed non-lesional skin biopsies [7], the lupus band test in fully photoprotected non-lesional skin, such as buttocks or the inner arm consisting of three or more components (IgA, IgG, IgM or complement proteins) is positive in 55% of cases [7], and appears to have the highest specificity for SLE than any other test [7,14], it can be used for diagnosis when clinical and serological criteria are insufficient [15].
A positive lupus band may also serve as a prognostic indicator in patients with an established diagnosis of LE [7]. If it is positive in non-injured photoprotected skin, it indicates less long-term survival [9,10] Some authors suggest a significant relationship between a positive lupus band in non-lesional skin and renal pathology, since 70% of these patients have active nephritis, being severe renal disease 3 times more common [12]. Patients with pure IgM deposits in non-lesional skin have anti-DNA antibodies restricted to the IgM class and tend to have a less severe course of disease. On the other hand, the deposits of C1q along the DEJ in the skin of patients with SLE may reflect the presence of DNA in the DEJ, these patients have a high rate of disease activity [7]. Also, positivity in non-lesional photoprotected skin indicates less long-term survival [9,10].
Drug-induced Lupus Erythematosus
Older age at onset and leukocytoclastic vasculitis are more commonly seen in drug-induced LE, mucin deposition and positive immunofluorescence are clues to the idiopathic form [19,20].
Neonatal Lupus Erythematosus
DI is not performed on a routine basis on NLE lesions, diagnosis is based on characteristic skin lesions with or without evidence of heart block, as well as serological markers of the disease in infants, their mothers, or both. It has been noted that immune deposits in NLE are also found throughout the DEJ [21,22],the most frequent are IgG, IgM and C3. In a clinical and histopathological analysis of 10 infants with NLE, the authors noted that only two biopsies were analysed with DI, and from them, only one case turned out positive [21]. Other authors also report that approximately 50% of the biopsies have positive DI [23].
Lupus Tumidus
Results of DI in patients with LET are mostly negative at the dermoepidermal junction or around the papillary and reticular dermal blood vessels, a study where 40 LET cases were evaluated could not found none of the 3 major immunoglobulin classes (IgG, IgM, and IgA) or complement components (C3 and C4) in any specimen, only 10% of patients were ANA positive [24].
Lupus Band Test Definition
In 1963, Burnham et al., described for the first time the deposits of immunoglobulins in the DEJ along with a thickened basement membrane in the lesional skin of patients with SLE [6]. Subsequently, Cormane demonstrated similar deposits in clinically normal skin in patients with SLE [7].
The definition of positive lupus band test is controversial, by some authors it is defined as the presence of a bright fluorescent band in the DEJ [8]; while other authors consider as criteria the presence of IgM or IgG deposition (individually or in combination with another class of immunoglobulins) in the area of the basement membrane where complement components may be present [4]. Other authors describe that, to increase the specificity of the test, the presence of IgM alone (with or without C3) should be excluded from the definition of positive lupus band, since IgM is commonly found in patients with normal skin without SLE, especially in photoexposed skin, where weak deposits are describe [5,7]. IgG is the most specific immunoglobulin for LE and the most frequently reported, followed by IgM and IgA [9]. Another criterion that has also been mentioned is that to consider a positive lupus band test, the deposit of IgM in the photoexposed skin must be a continuous band that covers at least 50% of the sample width of the biopsy and be at least of moderate intensity, since mild IgM deposition throughout the DEJ is common in patients who do not have LE, as in actinic keratosis and rosacea lesions, and in 20%-25 of normal skin samples from healthy young adults; while only 5% show the presence of IgG, IgA or C2 [7,10] In non-photoexposed skin, an interrupted band of IgM of at least moderate intensity is sufficient to designate the test as positive [7].
Clinical Use of the Lupus Band Test
The sensitivity and specificity of the test is strictly related to the body area where the biopsy is performed and the criteria by which a positive value is assigned to the test. George et al., found that in DLE the test has a sensitivity of 58%, a specificity of 87%, a positive predictive value (PPV) of 95% and a negative predictive value (NPV) of 32%; for SLE, they reported a higher sensitivity of 93%, and a specificity of 87%, a PPV of 64% and a NPV of 98%.[5] In some studies it has had a specificity of up to 100% [25].
The sensitivity of the lupus band for active disease is higher than the serum levels of C3 and C4, anti-DNA antibodies, LE cells, lymphocyte count and ESR[19], its NPV and its high specificity, allows differentiating between LE and other skin disorders that are clinically similar [17,19] (Table 1).
The PPV for the lupus band test in SLE is greater with C4 (100%), properdine (91.3%), and IgA (86.2%) than with IgM (59%). Specificity and PPV also increase with the number of immunoreactive agents detected in the DEJ [7].
In DLE, although histopathological examination (HPE) and DI have a very high specificity and PPV (100%), DI has a higher sensitivity for diagnosis compared to HPE, and a higher NPV [11].
In a study that looked for the correlation of a positive lupus band with SLE activity measured with the SLEDAI score (Systemic Lupus Erythematosus Disease Activity Index), the frequency of the lupus band was found to be significantly higher in patients with active disease, as well as with higher titres of anti-dsDNA antibodies, and the presence of renal involvement [19].
Another diagnostic value that the test has is in the differential diagnosis of SLE from other diseases with positive antinuclear antibodies such as drug-induced LE, rheumatoid arthritis, scleroderma, dermatomyositis and mixed connective tissue disease [14].
A study found that CLE without SLE was the only subgroup with staining of solitary immunoreactant, while multiple staining was significantly associated with internal lupus [3], multiple rather than single immunoreactant on lesional skin may likely imply systemic involvement. Patients with positive DI have severe SLE, >1 immunoreactant on lesional skin correlates to higher immunological profile and SLE disease activity [26].
A study in India found that statistically significant risk of kidney involvement was presents both when patient had bullous lesions and DI positivity of unexposed skin [6]. DI from sun protected normal skin helps in assessing the severity of the disease and correlates positively with the risk of developing nephritis [6].
Lupus Band and Other Rheumatic Diseases
Occasionally, there is the presence of a positive lupus band test in the lesional skin of patients with dermatomyositis or systemic sclerosis, this finding is related to a more severe course of the disease [7].
A biopsy in skin with telangiectasias in scleroderma may demonstrate the presence of a band of low-intensity immunoglobulins [2]. Also, rare cases have shown weak dermoepidermal deposits in telangiectasias of patients with dermatomyositis, mainly IgA.
In rheumatoid arthritis there may be in rare cases dermoepidermal deposition of IgM, especially in cases where there is a high serum concentration. Bright bands of IgG throughout the dermoepidermal junction are present in cases with marked vascular damage [2].
Limitations – False Negatives and False Positives
Certain limitations of the test should be considered when interpreting the results. Although useful in diagnosis, the lupus band test is not considered a pathognomonic sign of SLE [17,20]. About one-third of patients with positive DI do not have SLE [5].
False negatives are often seen when there are levels of extravascular dermal IgG [14] or if biopsy is taken from non-photo-exposed sites, where the lupus band is often absent despite SLE lesions, such as the trunk [27].)
Elbendary et al., found that IgM deposits showed greater sensitivity for the diagnosis of LE when they were found at the stromal-epithelial junction (SEJ) of the sweat glands and greater specificity when detected along the SEJ of hair follicles [28] when compared with other immune mediated diseases, also, the pattern of IgM in lupus and dermatomiositis is granular, in contrast to the linear deposition in other autoimmune disorders [28]. This may be helpful in narrowing differential diagnosis when evaluating DI specimens of immune-mediated dermatoses. Full thickness skin biopsy specimens that demonstrate adnexal structures are preferred as they are more likely to demonstrate adnexal structures [28–31].
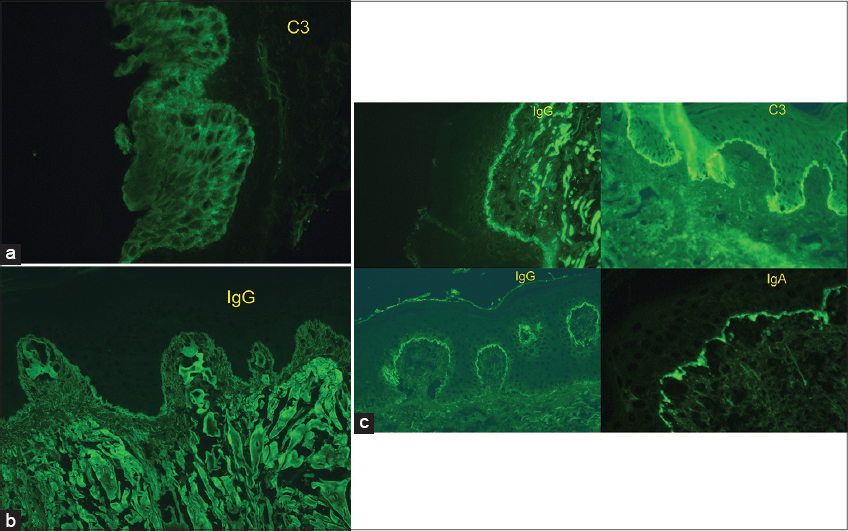
Finally, the lupus band test can be positive in non-autoimmune diseases such as porphyria cutanea tarda, and dermatitis herpetiformis (Figs. 3 and 4)
CONCLUSION
The lupus band test is a useful diagnostic tool in patients with LE, however, the correct interpretation of the test requires detailed knowledge of the patient’s context, such as the site of the biopsy, the components of the immunoreactants found in the DEJ, the morphology and intensity of the immunofluorescent band and other serological findings, as well as the response to treatment. A positive test in non-lesional photoprotected skin represents a criterion with high specificity to identify patients with SLE. The lupus band test is a procedure that can be useful in the diagnosis of LE and must be interpreted according to clinical findings, serological and immunological parameters in order to achieve a correct diagnosis.
ACKNOWLEDGEMENTS
We want to acknowledge the support we had from Dr. Sally Self from the pathology department at MUSC, all the immunofluorescence microphotographs are reproduced with her permission. All authors revised the manuscript and provided critical feedbacks. All authors approved the final version of the manuscript for submission.
REFERENCES
1. Maidhof W, Hilas O. Lupus:An overview of the disease and management options. P T. 2012;37:240-9.
2. Harrist TJ, Mihm MC. The specificity and clinical usefulness of the lupus band test. Arthritis Rheum. 1980;23:479-90.
3. Chanprapaph K, Tankunakorn J, Suchonwanit P, Rutnin S. Dermatologic Manifestations, Histologic Features and Disease Progression among Cutaneous Lupus Erythematosus Subtypes:A Prospective Observational Study in Asians. Dermatol Ther (Heidelb). 2021;11:131-47.
4. Sinem Ba?c?I, Aoki R, Vladimirova G, Ergün E, Ruzicka T, Sárdy M, et al. New-generation diagnostics in inflammatory skin diseases:Immunofluorescence and histopathological assessment using ex vivo confocal laser scanning microscopy in cutaneous lupus erythematosus. Exp Dermatol. 2021;30(5):684-690.
5. Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-24.
6. Ghosh AP, Nag F, Biswas S, Rao R, De A. Clinicopathological and Immunological Profile of Patients with Cutaneous Manifestations and their Relationship with Organ Involvement in Systemic Lupus Erythematosus Attending a Tertiary Care Center of Eastern India. Indian J Dermatol. 2020;65:22-8.
7. Reich A, Marcinow K, Bialynicki-Birula R. The lupus band test in systemic lupus erythematosus patients. Ther Clin Risk Manag. 2011;7:27-32.
8. Dahl MV. Usefulness of direct immunofluorescence in patients with lupus erythematosus. Arch Dermatol. 1983;119:1010-7.
9. Bharti S, Dogra S, Saikia B, Walker R, Chhabra S, Saikia U. Immunofluorescence profile of discoid lupus erythematosus. Indian J Pathol Microbiol. 2015;58:479-82.
10. George R, Mathai R, Kurian S. Cutaneous lupus erythematosus in india:immunofluorescence profile. Int J Dermatol. 1992;31:265-9.
11. Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus:a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409-15.
12. David-Bajar KM, Bennion SD, DeSpain JD, Golitz LE, Lee LA. Clinical, histologic, and immunofluorescent distinctions between subacute cutaneous lupus erythematosus and discoid lupus erythematosus. J Invest Dermatol 1992;99:251-7.
13. David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol 1993;100:2S-8S.
14. Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus:A review. J Cutan Pathol. 2001;28:1-23.
15. Sontheimer RD, Provost TT. Lupus erythematosus. In:Jordon RE, editor. Immunologic diseases of the skin. Norwalk (CT):Appleton &Lange;1991. 355-78.
16. Gilliam JN. The significance of cutaneous immunoglobulin deposits in lupus erythematosus and NZB/NZW F,hybrid mice. J Invest Dermatol 1975;65:154-61.
17. Dahl MV, Gilliam JN. Direct immunofluorescence in lupus erythematosus. In:Beutner EH, Chorzelski TP, Kumar V, editors. Immunopathology of the skin. 3rd ed.New York:John Wiley &Sons;1987. 499-518.
18. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-86.
19. Mehta V, Sarda A, Balachandran C. Lupus band test. Indian J Dermatol Venereol Leprol. 2010;76:298-300.
20. Guicciardi F, Atzori L, Marzano AV, Tavecchio S, Girolomoni G, Colato C, et al. Are there distinct clinical and pathological features distinguishing idiopathic from drug-induced subacute cutaneous lupus erythematosus?A European retrospective multicenter study. J Am Acad Dermatol. 2019;81:403-11.
21. Watson RM, Provost TT. Neonatal lupus erythematosus. In:Beutner EH,Chorzelski TP,Kumar V, editors.Immunopathology of the skin. 3rd ed.New York:John Wiley &Sons;1987. 583-610.
22. Vonderheid EC, Koblenzer PJ, Ming PML, Burgoon CF Jr. Neonatal lupus erythematosus. Arch Dermatol 1976;112:698- 705.
23. Maynard B, Leiferman KM, Peters MS. Neonatal lupus erythematosus syndrome. J Cutan Pathol. 1991;18:333-8.
24. Kuhn A, Richter-Hintz D, Oslislo C, Ruzicka T, Megahed M, Lehmann P. Lupus erythematosus tumidus–a neglected subset of cutaneous Lupus erythematosus:report of 40 cases. Arch Dermatol. 2000;136:1033-41.
25. Mysorekar V, Shyam Prasad A, Sumathy T. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172.
26. Luo YJ, Tan GZ, Yu M, Li KW, Liu YY, Guo Q, et al. Correlation of cutaneous immunoreactants in lesional skin with the serological disorders and disease activity of systemic lupus erythematosus. PLoS One. 2013;8:e70983.
27. Weigand DA. Lupus band test:Anatomic regional variations in discoid lupus erythematosus. J Am Acad Dermatol. 1986;14:426-8.
28. Elbendary A, Zhou C, Valdebran M, Yu Y, Gad A, Kwon EJ, et al. Specificity of granular IgM deposition in folliculosebaceous units and sweat gland apparatus in direct immunofluorescence (DIF) of lupus erythematosus. J Am Acad Dermatol. 2016;75:404-9.
29. Reimer G, Huschka U, Keller J, Kammerer R, Hornstein OP. Immunofluorescence studies in progressive systemic sclerosis (scleroderma) and mixed connective tissue disease. Br J Dermatol. 1983;109:27-36.
30. Jones SA, Black MM. The value of direct immunofluorescence as a diagnostic aid in dermatomyositis–a study of 35 cases. Clin Exp Dermatol. 1997;22:77-81.
31. Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-4923-8880 http://orcid.org/0000-0003-4923-8880 http://orcid.org/0000-0003-3312-5953 http://orcid.org/0000-0003-3312-5953 |




Comments are closed.