Two shades of malignancy in a psoriatic patient
Meryam Chaabani 1, Kahena Jaber1, Faten Rebhi1, Faten Gargouri2, Nejib Doss1, Mohammed Raouf Dhaoui1
1, Kahena Jaber1, Faten Rebhi1, Faten Gargouri2, Nejib Doss1, Mohammed Raouf Dhaoui1
1Department of Dermatology, Military Hospital of Tunis, Tunisia, 2Department of Pathology, Military Hospital of Tunis, Tunisia.
Corresponding author: Meryam Chaabani, MD
How to cite this article: Chaabani M, Jaber K, Rebhi F, Gargouri F, Doss N, Dhaoui MR. Two shades of malignancy in a psoriatic patient. Our Dermatol Online. 2021;12(4):436-438.
Submission: 02.12.2020; Acceptance: 24.02.2021
DOI: 10.7241/ourd.20214.20
Citation tools:
Copyright information
© Our Dermatology Online 2021. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Psoriasis is a chronic inflammation of the skin, affecting approx. 120 million people worldwide. The risk of developing skin cancer in psoriatic patients has been stressed, although with a significant amount of conflicting data in the literature. The most consistent findings suggest that psoriatic patients are at an increased risk of non-melanoma skin cancer. An increased risk of melanoma has not been well established. Herein, we report the case of a patient with a history of pustular psoriasis present since the age of ten, who developed an SCC and a melanoma, and discuss the etiopathology of this association.
Key words: Psoriasis; Melanoma; Squamous Cell carcinoma; Inflammation
INTRODUCTION
Psoriasis is a chronic inflammation of the skin, affecting approx. 120 million people worldwide. The association between psoriasis and a substantial increase in the risk of systemic and skin cancers has been debated increasingly often. The association between squamous cell carcinoma (SCC) and psoriasis is well established. Several cases of melanoma in psoriatic patients, however, have been reported in the literature. Herein, we report the case of a psoriatic patient who developed an SCC and a melanoma in the same area.
CASE REPORT
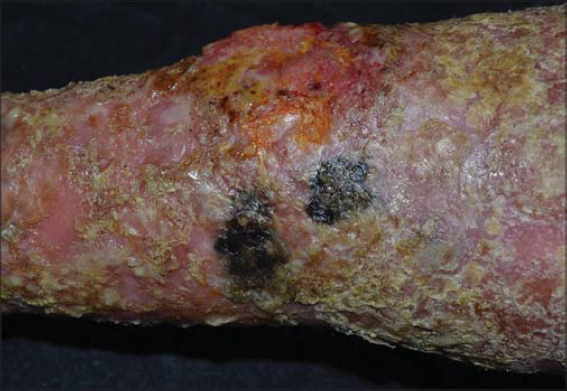
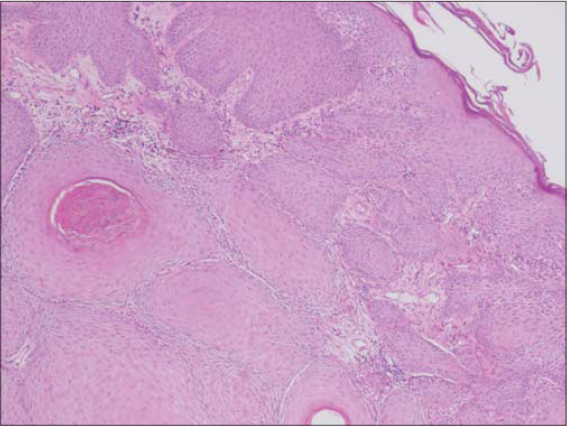
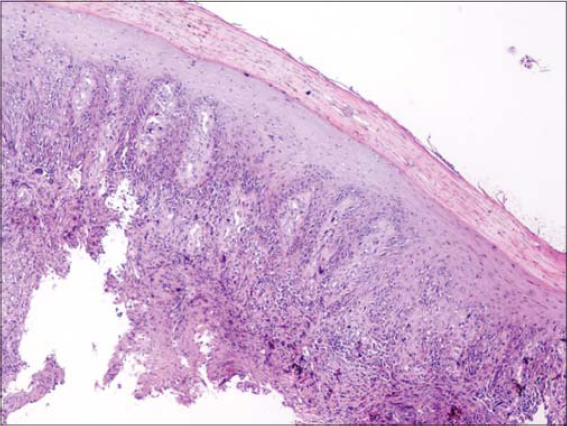
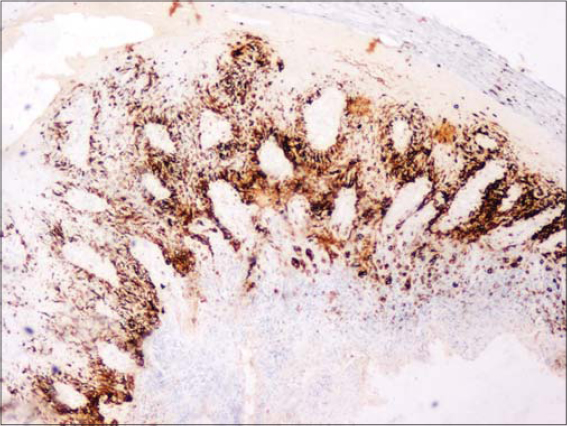
A fifty-year-old male with skin phototype IV was admitted to our department in December 2016 with a bleeding tumor on the right leg. The patient had a history of pustular psoriasis present since the age of ten. There was neither a history of intensive sun exposure nor a family history of skin cancers. The patient was treated for many years with acitretin and topical steroids. In 2010, the patient had extensive exacerbations requiring several hospitalizations. Acitretin was temporarily replaced with MTX, which was discontinued after two weeks due to anemia and digestive intolerance. Since then, the patient experienced severe involvement on the feet, legs, and arms. An examination revealed multiple erythematous plaques covered by yellowish scales on the upper and lower limbs. A bleeding and ulcerating plaque with everted edges and 6 × 5 cm in size was present on the anterior surface of the right leg (Fig. 1). The patient also had two irregular pigmented plaques on the inner side of the same leg 2 x 1.5 cm and 2 x 3 cm in size (Fig. 1). Skin biopsies of the lesions were performed, concluding to an invasive SCC (Fig. 2) and a superficial spreading melanoma corresponding to the pigmented plaques with a Breslow thickness of 1 mm and a mitotic rate of 1/mm²) (Fig. 3 and 4). A cervico-thoraco-abdominal CT scan revealed bilateral inguinal lymphadenopathy. The patient was referred to the surgical oncology department. Amputation of the leg was indicated, given the difficult healing of the pathological skin, but the patient refused to undergo this surgically invasive procedure.
DISCUSSION
Several factors may increase the risk of carcinogenesis in psoriatic patients, including chronic inflammation, immunosuppressive treatments, and UV therapies [1]. Inflammatory mediators may contribute to neoplasia by inducing proneoplastic mutations, resistance to apoptosis, and the stimulation of angiogenesis [2]. SCC has been reported to be associated with phototherapy, cyclosporine, and MTX [3,4]. As an immunosuppressive drug, MTX may inhibit cancer-related immune surveillance. Thus, drug-induced immunosuppression is a risk factor for malignancies, particularly squamous cancers [5]. The association between PUVA therapy and an increased risk of SCC is well established. A systematic review of 46 studies on PUVA therapy concluded that SCC may develop with low-level UVA exposure and the risk increases linearly with the number of treatments [6]. However, the risk of developing a melanoma in psoriatic patients remains unclear, with studies concluding to an increased risk [7,8] and others showing no increased risk when compared with cancer rates in the general population [3,9].
As for our patient, besides the chronic inflammation of psoriasis, there were no other risk factors for skin cancer. However, the patient was treated with acitretin for years, which could have been a protective factor against the development of skin cancer [10,11]. In this sense, chronic inflammation in psoriasis may be a precursor of skin cancer, mainly non-melanoma skin cancers. Numerous transcription factors and cytokines thought to play a role in psoriasis may also promote tumor development. These include interleukin (IL)-6, TNF-a, and signal transducer and activator of transcription (STAT)-3 [12]. Proinflammatory cytokines, such as TNF and IL-1, activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which is an intracellular transducer of inflammatory signals [13] whose activation may enhance tumor cell proliferation.
CONCLUSION
We reported the particular case of a psoriatic patient who developed two types of skin cancer: SCC and melanoma.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, Gelfand JM. The risk of cancer in patients with psoriasis:A population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-90.
2. Neagu M, Constantin C, Caruntu C, Dumitru C, Surcel M, Zurac S. Inflammation:A key process in skin tumorigenesis. Oncol Lett. 2019;17:4068-84.
3. Geller S, Xu H, Lebwohl M, Nardone B, Lacouture M E, Kheterpal M. Malignancy risk and recurrence with psoriasis and its treatments:A concise update. Am J Clin Dermatol. 2017;19:363-75.
4. Wang X, Liu Q, Wu L, Nie Z, Mei Z. Risk of non-melanoma skin cancer in patients with psoriasis:An updated evidence from systematic review with meta-analysis. J Cancer. 2020;11:1047-55.
5. Scott FI, Mamtani R, Brensinger CM, Haynes K, Chiesa-Fuxench ZC, Zhang J, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152:164-72.
6. Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maître M, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis:A systematic literature review. J Eur Acad Dermatol Venereol. 2012;26:22-31.
7. Bhattacharya T, Nardone B, Rademaker A, Martini M, Amin A, Al-Mudaimeagh HM, et al. Co-existence of psoriasis and melanoma in a large urban academic center population:A cross-sectional retrospective study. J Eur Acad Dermatol Venereol. 2016;30:83-5.
8. Chen YJ, Wu CY, Chen TJ, Shen JL, Chu SY, Wang CB, et al. The risk of cancer in patients with psoriasis:A population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
9. Hannuksela-Svahn A, Pukkala E, LääräE, Poikolainen K, Karvonen J. Psoriasis, its treatment, and cancer in a cohort of Finnish patients. J Invest Dermatol. 2000;114:587-90.
10. De Graaf YG, Euvrard S, Bouwes Bavinck JN. Systemic and topical retinoids in the management of skin cancer in organ transplant recipients. Dermatol Surg. 2004;30(4 Pt 2):656-61.
11. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma:management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-61.
12. Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42:161-70.
13. Beyaert R, Beaugerie L, Van Assche G, Brochez L, Renauld JC, Viguier M, et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol Cancer. 2013;12:98.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.