Dupilumab-induced facial erythema: Superior effect of a topical antifungal over oral
Amnah Almulhim , Yasir Shaikh, Lenah Shaikh, Ahad Shaikh
, Yasir Shaikh, Lenah Shaikh, Ahad Shaikh
Dermatology Department, King Fahad Specialist Hospital, Dammam, Saudi Arabia
Corresponding author: Amnah Almulhim, MD
How to cite this article: Almulhim A, Shaikh Y, Shaikh L, Shaikh A. Dupilumab-induced facial erythema: Superior effect of a topical antifungal over oral. Our Dermatol Online. 2021;12(3):294-296.
Submission: 02.01.2021; Acceptance: 26.04.2021
DOI: 10.7241/ourd.20213.15
Citation tools:
Copyright information
© Our Dermatology Online 2021. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Atopic dermatitis (AD) is a common disease that affects different age groups, ranging from mild to severe. For a patient with severe atopic dermatitis, systemic treatment options are limited and only one biological treatment is available: dupilumab. Dupilumab was initially approved by FDA for the treatment of adults with AD and recently approved for the treatment of adolescents with AD. As with approving other medications, some side effects are not reported before phase III trials. Thereby, we present a case of dupilumab-induced facial erythema treated with an oral and topical antifungal.
Key words: Dupilumab; Facial erythema; Redness; Atopic dermatitis
INTRODUCTION
Atopic dermatitis (AD) is a common chronic inflammatory disease with an incidence of 10–30% in the pediatric population and 2–10% in adults [1]. Treatment is usually aimed to reduce the frequency and duration of flares in addition to symptomatic treatment. Most treatments are topical, treating mild to moderate AD and, until recently, systemic options treating severe AD have been limited. Dupilumab is considered a revolutionary medication in the treatment of AD, approved by the FDA (Food and Drug Administration) in March 2017 for the treatment of AD in adults. Furthermore, it was the first biological treatment approved for the treatment of AD [2,3]. In March 2019, it was approved by the FDA for the treatment of AD in adolescents [2]. Dupilumab is a fully-humanized monoclonal antibody that works by blocking a shared alpha receptor in interleukin 4 (IL-4) and interleukin 13 (IL-3), leading to an inhibitory effect, thus suppressing T-helper 2 (Th2) inflammatory mediators, which are strongly involved in the pathogenesis of AD [4]. Dupilumab is considered a safe medication, with reported side effects that include local site reaction, antibody development, conjunctivitis, oral herpes simplex, arthralgia, and eosinophilia in order. Nevertheless, as with any other medication, some side effects are reported after phase III trials. Facial erythema was reported in several articles [5–10]. Herein, we report a case of facial erythema induced by dupilumab.
CASE REPORT
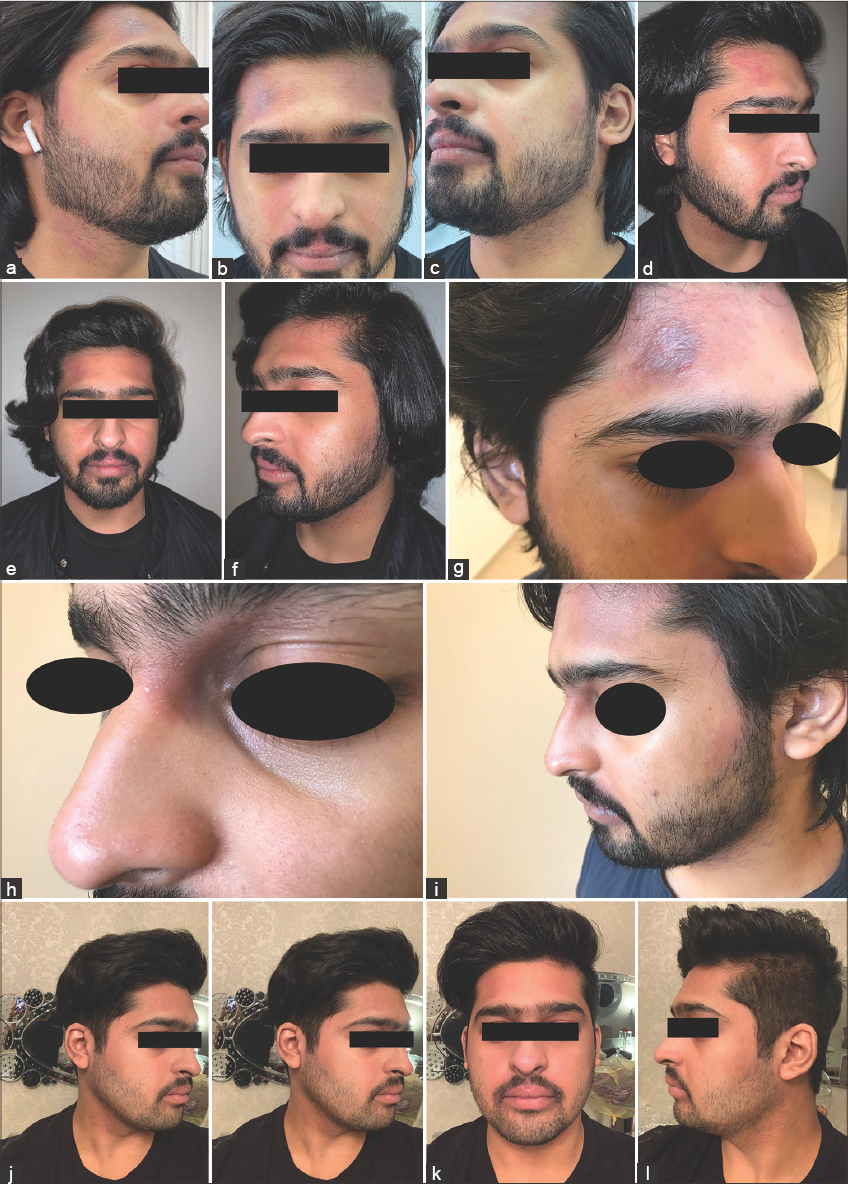
A 21-year-old male, a case of atopic dermatitis present since childhood, was managed initially with a topical steroid, to which he was partially responsive. The patient had extensive AD involving the entire body, including the face. He was given cyclosporin in 2018 with a good improvement. In August 2019, he was started on dupilumab—given its safety when compared with cyclosporin—with a loading dose of 600 mg subcutaneously (SQ), followed by 300 mg SQ every two weeks as an outpatient. The patient noted an improvement after the second dose. However, he reported the development of facial erythema. After two weeks of starting the medication, he experienced the exacerbation of the facial lesions, which unresponsive to topical steroids. On examination, ill-defined, non-scaly, dusky erythematous patches were present on the face and neck (Figs. 1a – 1c). After reviewing the literature, we encountered two similar cases responding well to oral itraconazole. Thus, the patient was started on itraconazole 200 mg PO every day for four weeks. After one week of starting itraconazole, the patient reported the improvement of the facial erythema (Figs. 1d – 1f). However, he had a relapse before finishing the course (Figs. 1g – 1i). He was, then, started on topical miconazole with hydrocortisone cream twice a day for fourteen days with complete clearance of facial lesions, with a persistent response several months after stopping miconazole and hydrocortisone cream (Figs. 1k – 1l).
In addition to the facial erythema, the patient had developed conjunctivitis and was seen in ophthalmology. This was managed with eye lubricants: ophthalmic drops with 2% hyaluronic acid TID X 2 months in addition to tobramycin with dexamethasone ophthalmic solution TID for three weeks.
DISCUSSION
Dupilumab targets the alpha subunit of IL-13 and IL-14, thereby inhibiting Th2 cytokines. It is used to treat Th2-mediated diseases, mainly atopic dermatitis and bronchial asthma. The side effects reported by the pharmaceutical company include antibody development, local site reaction, conjunctivitis, eosinophilia, insomnia, and herpes simplex infection among others [2,10]. Nonetheless, facial erythema is not reported before phase III trials. We report this case to support the already reported cases and to show the superior results of topical antifungals over oral antifungals.
The exact pathomechanism of developing facial erythema is unknown [7]. However, the Malassezia spp. are believed to be involved, given the response to antifungals and the elevated level of serum Malassezia-specific IgE. It was hypothesized that T-helper 17 (Th17) induced activation by dupilumab leads to the proliferation of the Malassezia spp. [6]. This might be counterargued, as the Malassezia spp. are part of healthy flora, yet the response to treatment supports this involvement. In our patient, the lesions were not scaly, thus scarping for a fungal culture was not done. Given the anatomic location, we preferred postponing the biopsy if no response was to be seen with oral and topical antifungals.
CONCLUSION
Based on our humble clinical experience, we suggest treating facial erythema with a topical antifungal initially, with a fungal culture from the scales if present. If no response is observed, we recommend adding oral itraconazole, thereby avoiding possible systemic side effects from oral itraconazole, with similar or even superior results. We also recommend delaying the biopsy, given the anatomical preference of this dermatosis, thus avoiding scars in a highly aesthetic area.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood:ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733-43.
2. DUPIXENT® (dupilumab) injection, for subcutaneous use. Package Insert. US Prescribing Information and Patient Information. 2019.
3. Mullard A. FDA approves dupilumab for severe eczema. Nat Rev Drug Discov. 2017;16:305.
4. Wang FP, Tang XJ, Wei CQ, Xu LR, Mao H, Luo FM. Dupilumab treatment in moderate-to-severe atopic dermatitis:A systematic review and meta-analysis. J Dermatol Sci. 2018;90:190-8.
5. de Beer FSA, Bakker DS, Haeck I, Ariens L, van der Schaft J, van Dijk MR, et al. Dupilumab facial redness:Positive effect of itraconazole. JAAD Case Rep. 2019;5:888-91.
6. de Wijs LEM, Nguyen NT, Kunkeler ACM, Nijsten T, Damman J, Hijnen DJ. Clinical and histopathological characterization of paradoxical head and neck erythema in patients with atopic dermatitis treated with dupilumab:A case series. Br J Dermatol. 2020;183:745-9.
7. Waldman RA, DeWane ME, Sloan B, Grant-Kels JM, Characterizing dupilumab facial redness:A multi-institution retrospective medical record review. J Am Acad Dermatol. 2020;82:230-2.
8. Albader SS, Alharbi AA, Alenezi RF, Alsaif FM, Dupilumab side effect in a patient with atopic dermatitis:A case report study. Biologics. 2019;13:79-82.
9. Zhu G A, Chen JK, Chiou A, Ko J, Honari G, Assessment of the development of new regional dermatoses in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155:850-2.
10. Ou Z, Chen C, Chen A, Yang Y, Zhou W. Adverse events of Dupilumab in adults with moderate-to-severe atopic dermatitis:A meta-analysis. Int Immunopharmacol. 2018;54:303-10.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.