Drug reactions with the involvement of the periungual folds
Patricia Chang 1, Pamela María Mancilla Gudiel2
1, Pamela María Mancilla Gudiel2
1Department of Dermatology, Hospital General de Enfermedades IGSS and Hospital Ángeles, Guatemala, 2Student elective on Dermatology, Hospital General de Enfermedades IGSS and Hospital Ángeles, Guatemala
Corresponding author: Dr. Patricia Chang
Submission: 07.04.2020; Acceptance: 10.08.2020
DOI: 10.7241/ourd.20212.23
Cite this article: Chang P, Mancilla Gudiel PM. Drug reactions with the involvement of the periungual folds. Our Dermatol Online. 2021;12(2):188-194.
Citation tools:
Copyright information
© Our Dermatology Online 2021. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Drug reaction in hospitalized patients has an incidence rate of 2–3% and can affect any organ of the body, including the skin and its appendages. Each of the components of the nail apparatus can be affected, and the clinical manifestation to be observed will depend on the condition of each component. When it comes to the periungual folds, fixed drug eruptions, Stevens–Johnson syndrome, and Lyell’s syndrome are the associated skin drug reactions. Periungual lesions can manifest themselves as a condition per se or arise from a drug reaction. Erythema, hemorrhage, necrosis, painful desquamation, edema, vesicles, and dyschromia are among the lesions that can develop. Other possible reactions include paronychia and the formation of pyogenic granulomas caused by drugs. Therefore, it is important to assess the periungual folds should any drug reaction occur.
Key words: Periungual fold; Drug reactions; Drugs; Paronychia; Pyogenic granuloma
INTRODUCTION
According to the World Health Organization, a drug reaction is defined as an “unintended effect that occurs at drug doses normally used in patients for the prophylaxis, diagnosis or treatment of their diseases.” Drug reactions can involve various organs and systems of the body, and the skin is the most frequently involved, sometimes accompanied by the involvement of its appendages, such as the nail apparatus, hair, and mucosa. As for the nail apparatus, all its components may be affected, but we will focus on the lesions that can be evidenced at the level of the nail folds caused by drug reactions or pharmacodermas, which have similarly been observed [1–4].
The proximal nail fold is crucial because it gives rise to the formation of the nail plate through the dorsal matrix in the segment below its ventral portion and because, together with the lateral folds, it adheres to the dorsal surface of the nail plate and acts as a waterproof barrier that protects the nail matrix from injury and provides support and protection to the nail plate [1,5].
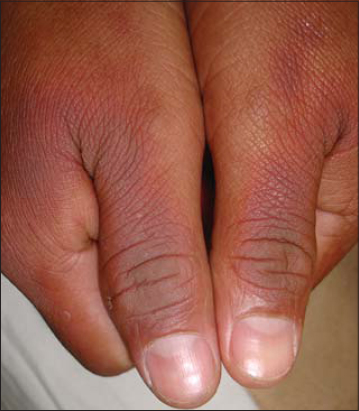
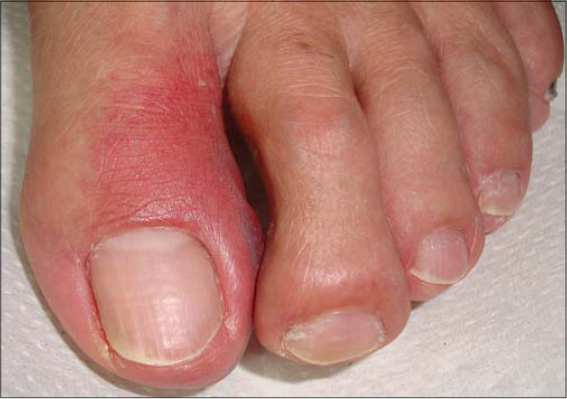
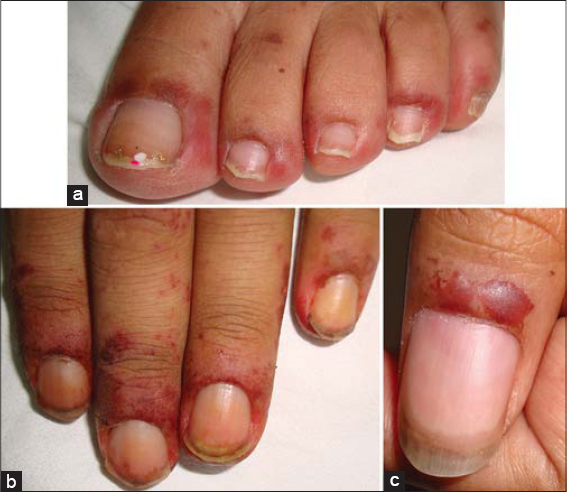
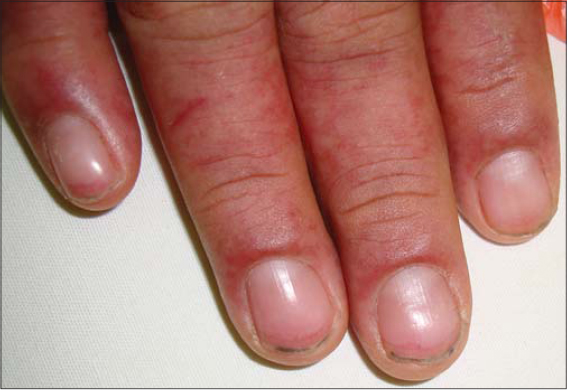
Pharmacodermas that may compromise the periungual folds include pigmented fixed erythema (Figs. 1 and 2), minor multiform erythema, Stevens–Johnson syndrome (Figs. 3a –3c), and Lyell’s syndrome (Fig. 4) [1,2]
The skin manifestations that are due to the different drug reactions already mentioned can lead to injuries such as erythema, bleeding, necrosis, desquamation, purpuric lesions, vesicular bullous lesions, epidermal exfoliation, and dyschromia (Fig. 5) (Table 1).
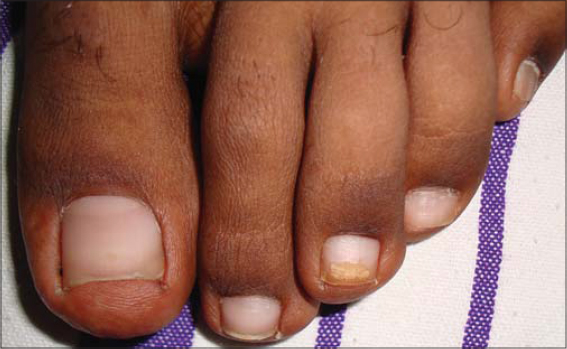 |
Figure 5: Acute and chronic paronychia from cetuximab in a patient undergoing treatment for head and neck cancer (primary unknown). |
 |
Table 1: Injuries to the periungual folds due to drug reactions |
Numerous case reports have been published with periungual tissues affected by drugs or components of drugs, such as capecitabine, which has been approved for the treatment of metastatic colorectal cancer and advanced breast cancer, and brings about adverse events that can affect the nail matrix, the nail bed, and the nail folds. These can manifest themselves as subungual hyperkeratosis, onycholysis, onychomadesis, acute paronychia, secretion of nail exudate, and vascular lesions in the nail folds. In the treatment of psoriasis with adalimumab, the paradoxical development of de novo psoriasis has appeared in some cases, especially the form called palmoplantar pustulosis. In another case report, nail changes occurred in all of the twenty nails, and the surrounding tissues were affected, displaying erythema, edema, scales, and inflammation of the proximal nail fold. In contrast, bleomycin as such produces no adverse events at the level of the nail folds; however, vascular changes may develop with the use of bleomycin near or around the periungual area. These changes can appear as Raynaud’s phenomenon or sclerodermiform changes, which can be acute and lead to gangrene and necrosis of the toes, requiring immediate cessation of treatment and its conclusive abandonment [1,2,6–10].
Drug-induced nail abnormalities are usually temporary and reversible once the causative agent is removed, and are rarely life-threatening. They frequently appear as paronychia and pyogenic granulomas (Table 2) [2,11–14].
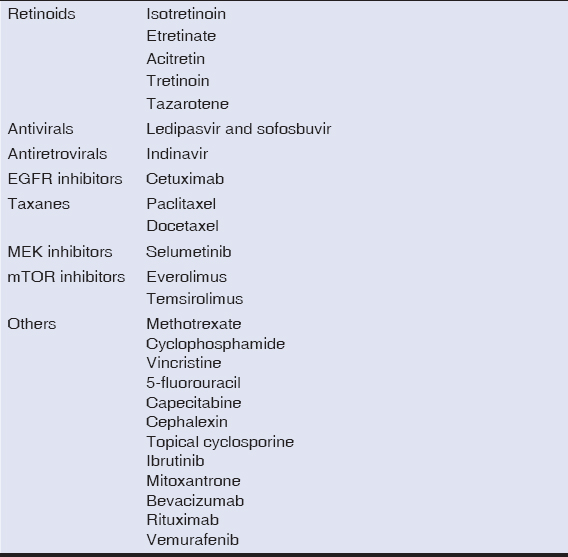 |
Table 2: Drugs that most frequently induce paronychia and/or pyogenic granulomas |
Paronychia and Pyogenic Granuloma
Paronychia is an inflammation of one or more periungual folds that involves erythema, edema, and tenderness. In addition, it can be exceptionally painful and can restrict fine motor activity. It can be acute, or chronic if the inflammation persists for more than 6 weeks. Drug-induced paronychia mainly affects the thumbs and the first toe, and occurs shortly after starting treatment [7,11,15–17].
Paronychia can progress to form friable granulation tissue in the periungual folds and pyogenic granuloma lesions that later mimic onychocryptosis [10,13,18].
An important feature of drug-induced pyogenic granulomas is that they affect multiple fingers and/or toenails, but the location in the feet is more prevalent, possibly due to chronic footwear friction [3,7,19].
Generally, these lesions resolve with drug discontinuation, although this is often not a viable option since it may be secondary to the use of antineoplastic drugs. Some other therapeutic measures will be presented later in case drug discontinuation is not possible [20,21].
Retinoids
Retinoids can cause paronychia and pyogenic periungual granulomas approx. 3 months after starting treatment. These lesions are a known, although rare, adverse event of systemic retinoids, especially isotretinoin and etretinate. Chronic paronychia and granulation tissue formation in the lateral nail folds are most often seen with the use of etretinate and are less common with acitretin. Pyogenic granulomas may also appear at the site of topical retinoid application, as with tretinoin and tazarotene [7,8,20,22,23].
Retinoids have been postulated to induce these lesions as a result of weak bonding between keratinocytes, inducing nail fragility and leading to the penetration of nail plate fragments into the surrounding tissues. This, in turn, creates an inflammatory reaction against a foreign body. Furthermore, retinoids have angiogenic properties and inhibit collagenases and gelatinases in vitro [7,8,21].
Topical treatment with steroids and antimicrobials is recommended; a 2–3 week regimen of 2% topical mupirocin cream can be administered in the morning and 0.05% clobetasol propionate ointment in the evening. For lesions that persist despite retinoid removal or that show no response to topical treatment, a biopsy should be performed to exclude malignancy before using more aggressive treatment modalities, such as excision or destruction of the lesion [7,8,20,24].
Antivirals and antiretrovirals
Ledipasvir and sofosbuvir are a new class of direct-acting antivirals against the hepatitis C virus (HCV), and paronychia has currently been reported as a side effect of this treatment [21].
Antiretroviral therapy causes pyogenic granulomas in approx. 6% of patients, and the onset time is 2 months to 1 year from the start of treatment. Indinavir, a protease inhibitor, is the most common cause of chronic paronychia in patients infected with the human immunodeficiency virus (HIV). Protease inhibitors are believed to have a retinoid-like effect due to homologous portions of the amino acid sequences of cellular retinoic acid-binding protein 1 (CRABP1) and the catalytic site of HIV-1 protease. Therefore, retinoid receptors can be occupied and activated by an antiretroviral drug, thus increasing the activity of vitamin A and its analogs [7,8,16,21].
The treatment of periungual lesions induced by these drugs is similar to that of retinoids, involving topical and antimicrobial steroids. In the case of antiretrovirals, substitution with an alternate antiretroviral agent should be made whenever possible, since it frequently leads to the resolution of the condition. Should no response occur, an effective combination therapy of an ointment steroid and chemical phenol matrixectomy is recommended [8,15,16,21].
Epidermal growth factor receptor (EGFR) inhibitors
EGFR inhibitors, a form of targeted cancer therapy, offer a new possibility for the treatment of multiple types of advanced cancer, including metastatic non-small cell lung cancer and colorectal, breast, head, neck, and pancreatic cancer. Two classes of EGFR inhibitors are currently employed: monoclonal antibodies (cetuximab, panitumumab, and matuzumab), which target the extracellular binding domain; and small molecules that inhibit the activity of tyrosine kinase (gefitinib, erlotinib, lapatinib, and afatinib), which target the intracellular domain [7,12,25,26].
Despite the promising treatment efficacy of EGFR inhibitors as an antineoplastic drug and their better safety profile than conventional chemotherapy, their use is accompanied by numerous adverse skin events, leading to poor adherence to treatment, decreased dosage, or complete discontinuation of such treatment, in addition to the patient’s psychosocial discomfort. This is because EGFRs are crucial for the sound development and physiology of the skin, since they are expressed in the keratinocytes of the basal and suprabasal layers of the epidermis and the outer root sheaths of hair follicles. Inhibition of EGFR-dependent pathways leads to changes in epidermal cell differentiation and migration, in addition to inhibited keratinocyte proliferation and decreased cell survival through induction of apoptosis [8,10,12,18,25,27].
Cutaneous manifestations appearing in targeted therapies are found in more than 50% of patients under treatment, and are enumerated by the acronymic name of PRIDE syndrome: papulopustules and/or paronychia, regulatory abnormalities of hair growth, itching, and dryness. The earliest and most common adverse event is papulopustular or acneiform eruption observed in 50% to 100% of these patients. Periungual lesions occur less frequently. A 2012 meta-analysis estimated them at 17.2% with a relative risk of 76.94%. Even though dermatological symptoms can lead to a reduction in the quality of life of patients, it has been observed that the presence and severity of this toxicity have a positive correlation with the patient’s chance of survival and are considered a marker of tumor response [12,13,25,26,28].
Paronychia and pyogenic granulomas occur in 10% to 15% of patients under treatment. Paronychia develops approx. 1 to 2 months after starting EGFR inhibitors, but its pathogenesis remains unsettled. The epidermis is believed to thin and subsequently perforate the periungual tissue along the edges of the nail plate and induce an inflammatory foreign body reaction. Cetuximab is the most frequent targeted anticancer treatment causing paronychia (Fig. 6), corresponding to 10% to 20% of cases. As for the development of pyogenic granulomas, these are dose-dependent and usually show after six weeks of therapy. Resolution of this type of lesion occurs 1 to 2 months after drug withdrawal [7,8,10,11,14].
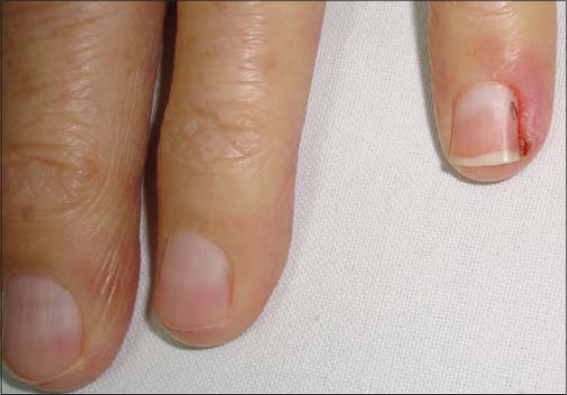 |
Figure 6: Proximal fold dyschromia in a patient undergoing treatment for seminoma. |
In the treatment of paronychia, the use of systemic tetracycline antibiotics, such as doxycycline, the combined application of powerful steroids and topical antiseptics, the management of secondary infections with antibiotics, and surgical intervention in patients with lateral matrixectomy, with phenolization in the nails affected, are described. Topical application of 0.1% adapalene gel daily has also been reported; the mechanism of action by which this topical retinoid alleviates the symptoms of paronychia involves its ability to increase the levels of EGF-type growth factor, which binds to heparin (HB-EGF; heparin-binding epidermal growth factor-like growth factor) and amphiregulin, which, in turn, reactivates the EGFR pathways in the epidermis and reduces periungual inflammation [7,8,16,26,29].
It has been mentioned that, in tyrosine-kinase-inhibitor–induced paronychia, a dose reduction can lead to some lesion improvement. However, a study that quantified the concentrations of these inhibitors on a paronychia site found no relationship with the degree of toxicity or the severity of the injury. One recommendation, hence, is to educate the patient on the measures to avoid finger compression, since it is a more effective approach to managing this adverse event than reducing the dose or discontinuing tyrosine-kinase-inhibitor anticancer drugs [18].
The management of pyogenic granulomas is more difficult than that of paronychia, and liquid nitrogen, topical or intralesional steroids, or weekly application of 10% aqueous silver nitrate, which reduces the granulation tissue, can be used for treatment. This type of tissue can also be removed by electro-drying. Photodynamic therapy has been reported to be a good therapeutic alternative since it has been shown to have the potential to completely resolve lesions or, at least, produce significant and symptomatic improvement, in addition to being very well-tolerated by patients. Occasionally, a partial phenol matrixectomy or treatment dose adjustment should be made if lesions are acutely painful, multiple, or persistent [10,13].
Recently, the use of topical beta-blockers for the treatment of paronychia and/or pyogenic periungual granulomas induced by EGFR inhibitors has been investigated. The results demonstrated that 0.5% timolol gel applied twice a day in occlusion for one month is a promising and safe treatment well tolerated by patients [29,30].
Taxanes
Taxanes—specifically, paclitaxel and docetaxel (Figs. 7a and 7b)—are commonly used in the treatment of breast, lung, and genitourinary cancers. Nail changes caused by these drugs have been reported in 40% to 89% of patients, manifesting themselves most often as onycholysis and discoloration of the nails and less frequently as acute paronychia. Capriotti et al. determined the general incidence of acute paronychia caused by these agents by a review of published clinical trials, and estimated it at 38.9% (1,481 / 3,812) [7,23,31].
 |
Figure 7: (a-b) Periungual fold dyschromia of the twenty digits accompanied by melanonychia. |
It has recently been reported that the use of 2% polyvidone iodine in dimethyl sulfoxide solution applied twice daily for 4 to 8 weeks is an effective topical treatment for paronychia induced by EGFR inhibitors and taxanes [32].
MEK, mitogen-activated ERK (extracellular signal-regulated kinase) activating kinase inhibitors
Periungual lesions have been observed in the use of MEK inhibitors. The frequency and severity of the condition are slightly less than of the lesions caused by EGFR inhibitors. Selumetinib, a member of the second generation of this group of drugs, induces paronychia, which is characterized by a late onset and resolution of lesions until drug discontinuation. According to one study, this adverse event appears in 9% of patients treated with selumetinib [10,23].
mTOR (mammalian Target of rapamycin)
mTOR inhibitors, such as everolimus and temsirolimus, induce periungual lesions in 15% of cases and are similar to EGFR and MEK inhibitors. This suggests that paronychia may be associated with a signaling inhibition of the pathways of mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K; phosphoinositide 3-kinase). Additionally, mTOR regulates the cellular signaling pathway of EGF, which could explain the appearance of pyogenic granulomas with this therapy [5,10,33].
Other drugs reported to have the potential to cause paronychia and/or periungual pyogenic granulomas are methotrexate, cyclophosphamide, vincristine, 5-fluorouracil, capecitabine, cephalexin, topical cyclosporine, ibrutinib, mitoxantrone, bevacizumab, rituximab, vemurafenib, and sorafenib (Fig. 8) [1,7,11,21,22,34,35].
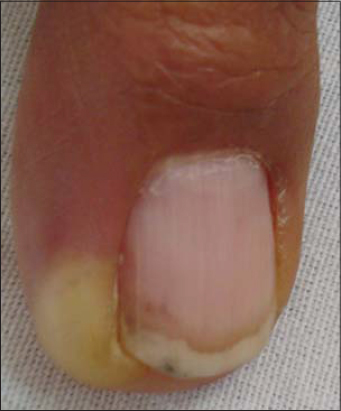 |
Figure 8: Paronychia from sorafenib in a patient undergoing kidney cancer treatment. |
General Measures
These injuries can become particularly debilitating, lead to functional impairment, and significantly affect the quality of life of patients under such treatment. Patients should be advised to use emollient lotions, wear gloves for housework and cleaning and frozen gloves and socks during the infusion of the drug, avoid impact, friction, and compression to the fingers, cut nails properly, use wide footwear, and take antiseptic baths, which can prevent painful periungual injuries [8,10,11,32,36].
ACKNOWLEDGMENT
We would like to thank Angela Alvarado Chew for translating this document.
REFERENCES
1. Chang P. Diagnosis using the proximal and lateral nail folds. Dermatol Clin. 2015;33:207-41.
2. Chang P. Drug reactions with involvement of the proximal nail fold. Skin Med. 2013;11:265-8.
3. Alonzo L, López L. Diagnóstico diferencial de reacciones medicamentosas adversas. Rev Cent Dermatol Pascua. 2000;9:120-5.
4. Chang P. Gálvez D. Reacciones medicamentosas con afección del pliegue proximal ungueal. Dermatol CMQ. 2012;10:172-7.
5. Patruno C, Balato N, Cirillo T, Napolitano M, Ayala F. Periungual and subungual pyogenic granuloma following anti-TNF-a therapy:is it the first case?Dermatol Ther. 2013;26:493-5.
6. Walker G, Lane N, Parekh P. Photosensitive lichenoid drug eruption to capecitabine. J Am Acad Dermatol. 2014;71:e52-3.
7. Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618-26.
8. Piraccini BM, Bellavista S, Misciali C, Tosti A, de Berker D, Richert B. Periungual and subungual pyogenic granuloma. Br J Dermatol. 2010;163:941-53.
9. Mishra V, Daniel RC, Elmets CA, Levin A, Elewski BE. Palmoplantar pustulosis with fulminant dystrophic 20-nail psoriasis in a patient receiving adalimumab therapy. J Drugs Dermatol. 2013;12:16-7.
10. Robert C, Sibaud V, Mateus C, Verschoore M, Charles C, Lanoy E, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16:e181-9.
11. Patel S, Tosti A. An overview of management of drug-induced hair and nail disorders. Clin Pract. 2014;11:327-39.
12. Korzycka M, Osmola-Mańkowska AJ, Bowszyc-Dmochowska M, Żaba RW, Adamski Z. Dermatological adverse effects in a cancer patient treated with an EGFR inhibitor. Case report and literature review. Dermatol Rev. 2018;105:632-8.
13. Fabbrocini G, Annunziata MC, Donnarumma M, Cacciapuoti S, Marasca C, Tosti A. Photodynamic therapy for periungual pyogenic granuloma-like during chemotherapy:our preliminary results. Support Care Cancer. 2018;26:1353-5.
14. Ho PH, Lin IC, Yang X, Cho YT, Chu CY. Using a novel scoring system for paronychia related to oncologic treatments (SPOT) for assessing paronychia severity and its correlation with pain index and quality of life. J Eur Acad Dermatol Venereol. 2019;33:204-12.
15. Relhan V, Goel K, Bansal S, Garg VK. Management of chronic paronychia. Indian J Dermatol. 2014;59:15-20.
16. Shemer A, Daniel III CR. Common nail disorders. Clin Dermatol. 2013;31:578-86.
17. Wollina U, Nenoff P, Haroske G, Haenssle HA. The Diagnosis and Treatment of Nail Disorders. Dtsch Arztebl Int. 2016;113:509-18.
18. Masago K, Irie K, Fujita S, Imamichi F, Okada Y, Katakami N, et al. Relationship between paronychia and drug concentrations of epidermal growth factor receptor tyrosine kinase inhibitors. Oncology. 2018;95:251-6.
19. Alessandrini A, Starace M, Piraccini BM. Dermoscopy in the evaluation of nail disorders. Skin Appendage Disord. 2017;3:70-82.
20. Arias-Santiago S, Husein-ElAhmed H, Aneiros-Cachaza J, Aneiros-Fernández J, Fernandez-Pugnaire MA. Uncommon side effects of isotretinoin therapy:Paronychia and pyogenic granuloma. J Am Acad Dermatol. 2011;64:AB37.
21. Sampson B, Lewis BKH. Paronychia associated with ledipasvir/sofosbuvir for hepatitis C Treatment. J Clin Aesthet Dermatol. 2019;12:35-7.
22. Piraccini BM, Venturi M, Patrizi A. Periungual pyogenic granulomas due to topical tazarotene for nail psoriasis. G Ital Dermatol Venereol. 2014;149:363-6.
23. Wollina U. Systemic drug-induced chronic paronychia and periungual pyogenic granuloma. Indian Dermatol Online J. 2018;9:293-8.
24. Benedetto C, Crasto D, Ettefagh L, Nami N. Development of periungual pyogenic granuloma with associated paronychia following isotretinoin therapy:a case report and a review of the literature. J Clin Aesthet Dermatol. 2019;12:32-6.
25. Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors:a review of cutaneous adverse events and management. Dermatol Res Pract. 2014;2014:1-8.
26. Hachisuka J, Yunotani S, Shidahara S, Moroi Y, Furue M. Effect of adapalene on cetuximab-induced painful periungual inflammation. J Am Acad Dermatol. 2011;62:e20-1.
27. Jin F, Zhu H, Kong L, Yu J. A spectrum of cutaneous toxicities from erlotinib may be a robust clinical marker for non-small-cell lung therapy:a case report and literature review. Onco Targets Ther. 2015;8:943-6.
28. Thakur V, Daroach M, Kumaran MS. Papulopustules and paronychia in a lung carcinoma. Eur J Inter Med. 2019;62:e5-6.
29. Lacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 2018;19:S31-9.
30. Sibaud V, Casassa E, D’Andrea M. Are topical beta-blockers really effective “in real life“for targeted therapy-induced paronychia. Support Care Cancer. 2019;27;2341-3.
31. Capriotti K, Capriotti J, LaCouture M, Lessin S, Belum V. Paronychia requiring intervention in patients receiving taxanes:A review of the literature. J Am Acad Dermatol. 2014;70:AB118.
32. Capriotti K, Capriotti J, Lessin S. Povidone-iodine nail solution is active in treating chemotherapy-associated paronychia. J Am Acad Dermatol. 2015;72:AB115.
33. Sibaud V, Dalene F, Mourey L, Chevreau C. Paronychia and pyogenic granuloma induced by new anticancer mTOR inhibitors. Acta Derm Venereol. 2011;91:584-5.
34. Mohandas D, Davis M. Paronychia and excess granulation tissue with ibrutinib:A newly reported side effect. J Am Acad Dermatol. 2016;74:AB222.
35. Piraccini BM, Iorizzo M, Tosti A. Drug-induced nail abnormalities. Am J Dermatol. 2003;4:31-7.
36. Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti-lung cancer therapies with EGFR-TKIs. Front Oncol. 2014;4:1-6.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.