|
|
Atopic dermatitis in adults from sub-Saharan Africa: epidemiological and clinical patterns, severity, and quality of life
Emmanuel Armand Kouotou 1,2, Jobert Richie Nansseu3,4, Eliane Fernande Minlo Nyangon1, Dahlia Nöelle Tounouga2, Defo Defo5, Coralie Reine Mendouga Menye6, Anne Cécile Zoung-Kanyi Bissek1
1,2, Jobert Richie Nansseu3,4, Eliane Fernande Minlo Nyangon1, Dahlia Nöelle Tounouga2, Defo Defo5, Coralie Reine Mendouga Menye6, Anne Cécile Zoung-Kanyi Bissek1
1Department of Internal Medicine and Specialties, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Yaoundé University Teaching Hospital, Yaoundé, Cameroon, 2Yaoundé University Teaching Hospital, Yaoundé, Cameroon, 3Department of Public Health, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Yaoundé, Cameroon, 4Department for the Control of Disease, Epidemics and Pandemics, Ministry of Public Health, Yaoundé, Cameroon, 5Yaoundé Central Hospital, Yaoundé, Cameroon, 6National Public Health Laboratory, Yaoundé, Cameroon
Corresponding author: Dr. Emmanuel Armand Kouotou
Submission: 15.05.2020; Acceptance: 15.08.2020
DOI: 10.7241/ourd.20211.1
Cite this article: Kouotou EA, Nansseu JR, Minlo Nyangon EF, Tounouga DN, Defo D, Mendouga Menye CR, Zoung-Kanyi Bissek AC. Atopic dermatitis in adults from sub-Saharan Africa: epidemiological and clinical patterns, severity, and quality of life. Our Dermatol Online. 2021;12(1):1-8.
Citation tools:
Copyright information
© Our Dermatology Online 2021. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Atopic dermatitis (AD) is a chronic and recurrent inflammatory dermatitis often associated with other atopic manifestations, such as asthma and allergic rhinitis. This study aimed to determine the epidemiological and clinical aspects of AD and to assess the quality of life (QoL) of patients suffering from AD in our setting.
Materials and Methods: This was a cross-sectional study conducted from February through April 2017 in seven hospitals in Cameroon. The study included patients above 18 who presented themselves to a dermatology consultation, were diagnosed with AD, and gave their consent. To assess the severity of AD and evaluate the QoL of the patients, standardized scales, such as SCORAD and QoLIAD, were employed.
Results: The study enrolled 46 patients between 18 and 69 years of age with a mean age of 31 ± 12 years and the prevalence of AD at 1.5%. Most of the participants were females, with a sex ratio of 0.4:1, living in urban areas (93.5%). Food (34.8%) and cosmetic products (21.7%) were found as the main risk factors in the occurrence of AD. Upon physical examination, the upper and lower limbs were found to be the most affected in 84.8% and 54.3% of cases, respectively; in addition to cutaneous xerosis (45.7%), lichenification (43.5%), and excoriations (37%). Of the 46 patients, 9 (20%) had severe AD, 32 (70%) had moderate AD, and 5 (10%) had mild AD. QoL was impaired in 43 of the 46 patients (93.5%).
Conclusion: Atopic dermatitis is a pathology that impacts the QoL of adults. A QoL assessment is, therefore, an important step in the management of AD.
Key words: Atopic dermatitis; Quality of life; Adults; Cameroon; Sub-saharan Africa
INTRODUCTION
Atopic dermatitis (AD) is a common chronic and pruritic skin condition characterized by progression through relapsing, whose long-term management can be difficult and daunting [1,2]. Its increasing prevalence and the overall cost of its treatment constitute a real public health problem in industrialized countries [1]. As its onset often falls onto infancy, AD is commonly seen in the practice of pediatric dermatology, beginning within the first year of life in 60% of cases and before the fifth year in 85% of cases [2].
The rapid increase in the prevalence of AD in the past twenty years can be due to many factors, such as industrialization, pollution, and reduced exposure to infectious agents. This observation derives from the “hygiene theory,” which claims that insufficient exposure to infectious agents in early childhood increases susceptibility to allergies and autoimmune diseases [3].
Most often reported in children, AD can also occur in adults, and its prevalence has, in recent decades, increased considerably in industrialized countries [4]. In general, AD affects 1–3% of adults worldwide, and can be either de novo in adults or continue from childhood into adulthood [5].
Furthermore, the chronic nature of AD and its evolution by relapsing over multiple years and its generation of itching can ultimately impact the quality of life (QoL) of both patients and their families [6–9].
Aside from a 2017 study by Kouotou et al. [9], which described a significant change in the QoL of children and their families brought about by AD, there have been none in Cameroon to evaluate the QoL of adults with AD. Because, to the best of our knowledge, data on AD in sub-Saharan Africa is lacking, we have conducted a study on Cameroonian adults suffering from AD in order to determine their epidemiological and clinical profiles and to assess the impact of AD on their QoL.
MATERIALS AND METHODS
Study Design and Setting
The following was a cross-sectional descriptive study conducted from February through April 2017 in the outpatient dermatology services of five health facilities in Yaoundé, Cameroon: Central Hospital of Yaoundé (CHY), Yaoundé Gynecology, Obstetrics and Pediatrics Hospital (HGOPY), University Teaching Hospital of Yaoundé (UTHY), Biyem-Assi District Hospital (BADH), Elig-Essono District Medical Center (EEDMC); and two health facilities in Douala, Cameroon: Laquintinie Hospital of Douala (LHD) and Douala General Hospital (DGH). These facilities were chosen based on the availability of experienced dermatologists providing dermatology consultations.
Study Participants
We included patients over 18 years of age who presented themselves to a dermatology consultation in one of the study facilities during the recruitment period with manifestations of AD and who gave their consent to participate in the study. Patients with other skin conditions that could impact the estimation of the severity of AD were excluded from the study. Participants were recruited consecutively and non-exhaustively during the period of the study.
The diagnosis of AD was made by clinical recognition using the criteria of the United Kingdom Working Party.
Procedure and Data Collection
We used a standardized data collection form to obtain information on sociodemographic (age, sex, place of residence, profession, level of education, marital status, profession) and clinical (sites and types of lesions) characteristics and to assess the severity of each case of AD and each patient’s QoL. Specific standardized scales were used to conduct these assessments. The evaluation of the severity of AD was performed during the consultation after the confirmation of the diagnosis. At the end of the process, the patient received a prescription, advice on the pathology, and an appointment date for a follow-up. Each patient’s QoL was assessed after the consultation.
To assess the severity of AD, we used a clinical scoring system for AD, specifically SCORAD (Scoring Atopic Dermatitis), whose measurement range is 0 to 103. The formula of the SCORAD index is as follows: (A / 5 + 7B) / (2 + C). Its classification of AD is as follows: mild (0–24), moderate (25–50), and severe (51–103).
A represents the extension of AD, which measures the affected surface by noting the extent of the patient’s inflammatory lesions and is calculated using the rule of nines (same as for burns). The total score ranges from 0% to 100%.
B represents six clinical features of varying disease intensities: erythema/darkening, edema/papules, oozing/crusting, excoriation, lichenification/prurigo, and dryness (evaluated on noninflamed skin), each on a scale of 0–3. The total score ranges from 0 to 18.
Finally, C represents the subjective symptoms as experienced by the patient, in particular daily pruritus and sleep loss, each on a scale of 0–10. The total score ranges from 0 to 20.
The QoL of each patient was assessed using the Quality of Life Index for Atopic Dermatitis (QoLIAD) [10], which is a scale that specifically assesses the impact that AD has on the QoL of a patient. QoLIAD comprises a questionnaire of 25 items intended for patients over 16 years old with answers either true (score = 1) or not true (score = 0) and scores from 0 to 25. The result is expressed as a percentage of the maximum possible score of 25. The higher the QoLIAD score, the greater and more significant the impact AD has on a patient’s QoL. Fig. 1 illustrates our process for recruiting our participants.
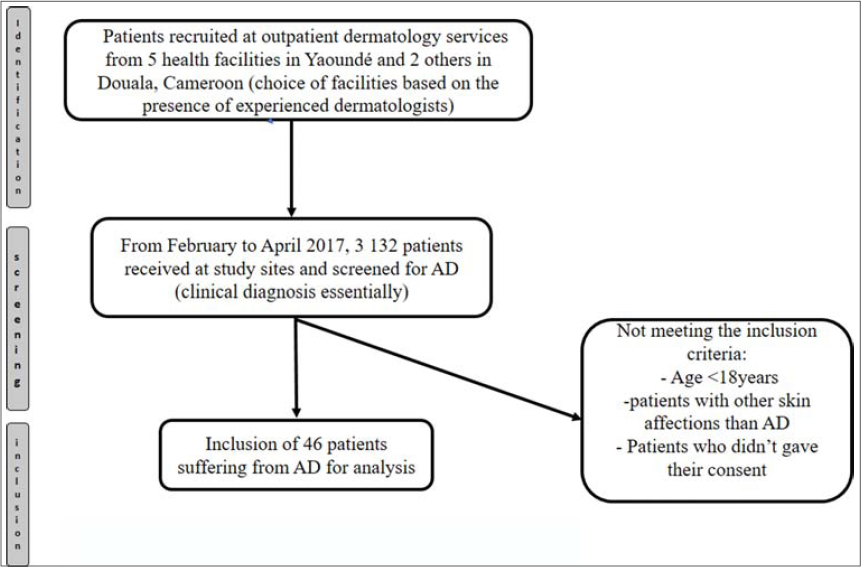
|
Figure 1: Flow diagram of the section “Materials and Methods.” |
Statistical Analysis
Data was coded and entered using the software CSPro and subsequently analyzed using SPSS Statistics, version 21.0, and R. Tables and figures were used to present the results, which were expressed as frequencies (percentages) of qualitative variables and means and standard deviations (SD) of quantitative variables. The comparison of the qualitative variables employed the Chi-square test or Fisher’s exact test, and the independence t-test or the one-factor were employed in the comparison of the quantitative variables where appropriate. Statistical significance was set at a p of 5%.
Ethics Statement
We conducted the study in accordance with national and international ethical rules and in accordance with the revised Declaration of Helsinki and the Council for International Organizations of Medical Sciences (CIOMS).
The study was granted ethical clearance by the Ethical Review Board of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I, Cameroon. In addition, we received administrative authorization from the directors of all hospitals comprehended by the study. The anonymity of the patients and the confidentiality of information gathered were respected.
The informed consent form was only obtained after signing the consent sheet by each of the patients. The study involved no major risk for the participants because no examination or invasive sampling was performed.
RESULTS
Prevalence of Atopic Dermatitis
From February through April 2017, 3132 patients visited the outpatient dermatology clinics of the selected hospitals. Of these 3132 patients, 46 suffered from AD and met the inclusion criteria, thus giving the prevalence of AD of 1.5% (95% confidence interval at 1.1–1.9).
Sociodemographic Characteristics of Patients
The age of the patients ranged from 18 to 69 years, with an average of 31 ± 12 years. There was a female predominance, with a sex ratio of 0.4/1. In addition, these patients were mainly single (65.2%), with a university education (73.9%), and resided mainly in urban areas (93.5%), as illustrated in Table 1.

|
Table 1: Sociodemographic characteristics of patients with atopic dermatitis (N = 46) |
Patient Medical History
The AD cases were either isolated (21/46; 45.7%) or associated with at least one other atopic condition (25/46; 54.3%), including allergic rhinitis (45.6%), asthma (8.7%), and allergic conjunctivitis (23.9%). Furthermore, a family history of atopy found in our patients concerned the ascendants, the collaterals, and the descendants (Table 2).
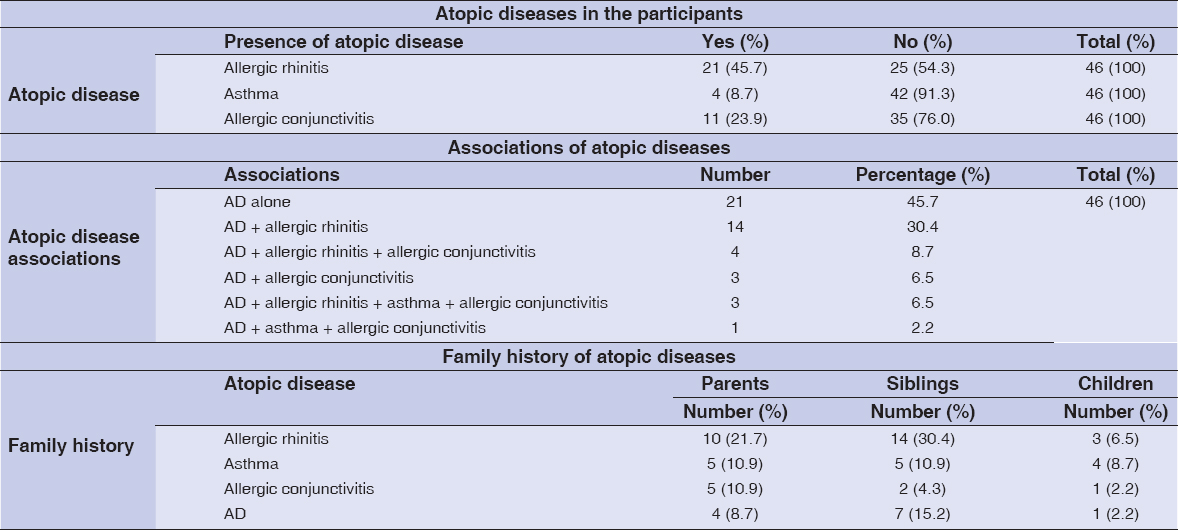
|
Table 2: Past medical history of the patients |
The risk factors in the occurrence of AD in the participants were dominated by food (34.8%) and the usage of cosmetic products (21.7%) (Table 3). Among foods, sugar (17.4%) and fish (13.0%) were the most incriminated; and perfumes (10.9%) were the first cosmetic products to be mentioned by the participants (Table 3).
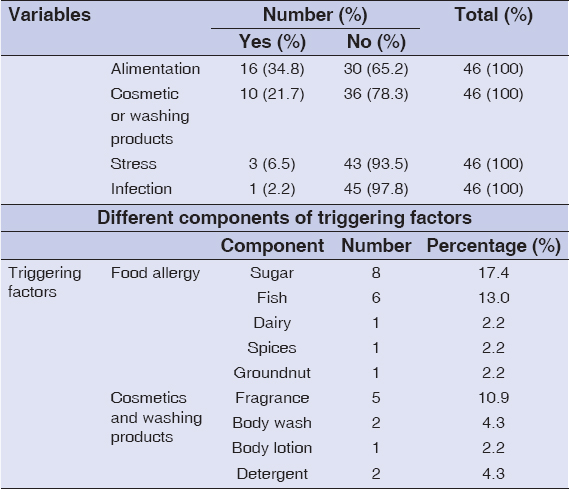
|
Table 3: Factors associated with atopic dermatitis |
The patients experienced an average of 1.7 ± 0.94 relapses of AD per year, with extremes varying from one to four relapses.
Pruritus was present in 43 of the 46 patients (93.5%), while 16 of the 46 patients (34.8%) reported a loss of sleep.
Clinical Data
Upon physical examination, the upper and lower limbs were found to be the most affected in 84.8% and 54.3% of cases, respectively. The elbows and popliteal fossa were the most affected areas in 35 (89.7%) and 16 (64.0%) patients, respectively. The physical signs were moderate skin dryness (45.7%), moderate lichenification (43.5%), and moderate excoriation (37%) (Table 4).
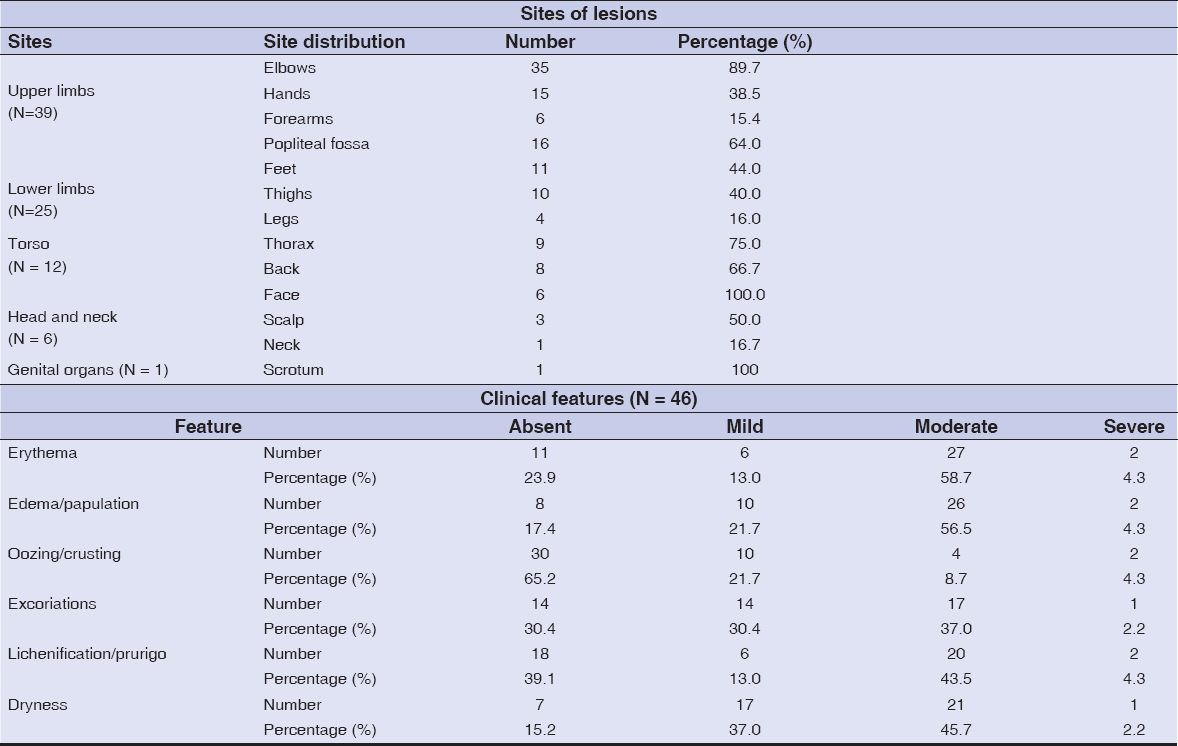
|
Table 4: Sites and types of lesions |
The severity of AD was classified according to the SCORAD score: mild (5/46; 10.9%), moderate (32/46; 69.6%), and severe (9/46; 19.6%) (Fig. 2).
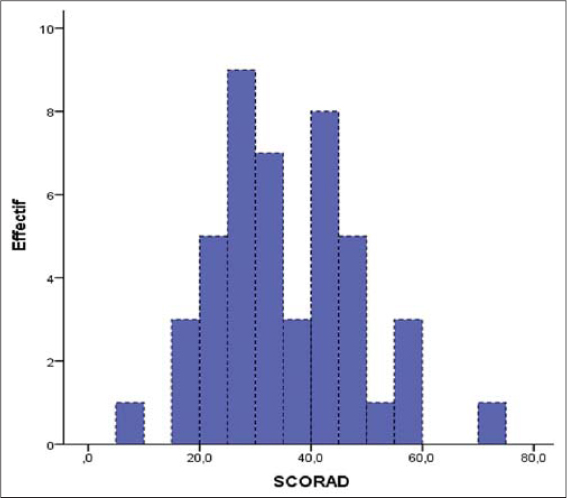
|
Figure 2: Distribution of patients according to SCORAD. |
Quality of Life
QoL was impacted in 43 patients (93.5%). QoL scores ranged from 1% to 20%. We, thus, found 9 (19.6%) and 34 (73.9%) patients whose QoL scores varied between 11% and 20% and between 1% and 10%, respectively. Furthermore, the score was 0% in 3 patients (6.5%) (Fig. 3).

|
Figure 3: Evaluation of QoL according to QoLIAD. |
The most incriminated factors in the changes in QoL concerned appearance: in particular, questionnaire items “I struggle with my appearance” (80.4%) and “I feel embarrassed about my skin appearance” (65.2%) (Table 5).

|
Table 5: Quality of life perception in patients suffering from AD (QoLIAD) |
DISCUSSION
The study took place from February through April 2017 in seven health facilities in the cities of Yaoundé and Douala with the aim to determine the epidemiological and clinical profiles of patients with AD, and to assess the impact of AD on their QoL. During the study’s time frame, 46 adult patients were diagnosed with AD.
Prevalence of Atopic Dermatitis
In our study, AD in adults represented 1.5% of the dermatology consultations, which is less than the 3.4% reported by Pesce et al. in Italy [11]. This might be explained by the fact that our sample was much smaller. Our prevalence might also be explained by the fact that AD is classically described as an uncommon condition in adults. Indeed, the prevalence of AD decreases as an individual matures from a child to an adult, according to the literature [12,13].
Sociodemographic Characteristics of Patients
The mean age was 31 ± 12 years, with extremes of 18 and 69 years. This result is close to the 30.4 years achieved by Zeppa et al. [14].
Women constituted 73% of the study population, with a sex ratio of 0.4. This might be explained by the fact that women, in general, exercise more apprehensiveness about their physical appearance and, thus, consult more readily, whether the pathology is visible or not. The same tendency has also been noted in Italy and India [5,11].
Our patients resided mainly (43/46; 93.5%) in urban areas, which is similar to the results obtained by Yemaneberhan et al. in Ethiopia [15]. In addition, a Chinese study highlighted a clear gradient in the prevalence of urban vs. rural AD: 10.2% vs. 4.6% [16]. The increased risk of atopy in urban areas might be explained by the industrialization of cities, air pollution, and various dietary habits [4,15,16].
Patient Medical History
In our study, we found that 54.3% of the patients had a personal history of allergic rhinitis, asthma, and allergic conjunctivitis. This result is close to that obtained by Ahogo et al. [2], who found that 54.2% of cases studied had a personal history of atopy.
Food allergy was the first risk factor in AD and accounted for 34.8% of cases. The relationship between AD and food allergy has been well-established in children, unlike adults, who are more sensitive to environmental allergens, such as molds, mites, and pollens [17,18].
Clinical Data
The upper limbs (84.8%) and lower limbs (54.3%) were the most affected, with a predominance in the elbows (96%) and popliteal fossa (64%). Zeppa et al. [14] found lesions to be located predominantly on the upper limbs (66.6%), followed by the face (45.9%) and the lower limbs (34.6%). In fact, these sites are described as the most affected in adults with AD [19] and these adults had lesions in at least three sites [20].
In our patients, the most common clinical signs were dry skin (45.7%), lichenification (43.5%), and excoriations (37.0%). The lichenoid aspect of the lesions might be due to the chronicity of the lesions and the intensity of the pruritus.
Moderate AD (69.6%) was the most common in our sample, which is higher than the 18.7% found by Zeppa et al. [14], who determined severe atopic dermatitis to be predominant (54.8 %).
Quality of Life
In our study, we employed QoLIAD, an AD scoring scale for adults [10], to find that QoL was impacted in 43 of all our 46 patients (93.5%). This result is close to the 88.6% noted by Cheok et al. [21]. Similar impact of AD on the QoL of patients, both children and adults, was discovered by other studies [7,9]. Health-related QoL is defined as a multidimensional construction comprised of impacts on different aspects of life, such as physical, emotional, mental, and social health, as well as daily functioning. In our case, changes in QoL can be explained not only by the chronic and relapsing nature of the pruritus but also by the sociocultural factors for which modern medicine can offer no definite solution.
In our study, we found a high percentage of positive responses to items in the QoLIAD questionnaire, mostly two: “I struggle with my appearance” (80.4%) and “I feel embarrassed about my skin appearance” (65.2%). This might be explained by the aesthetically displeasing nature of AD in a society in which appearance carries significant importance in interpersonal relationships and professional settings.
Nevertheless, our study, which concerned adults with AD, posed some limitations, in particular: 1) the fact that the sample was limited to hospital settings and, therefore, may not have been representative of the general population; 2) the choice of convenience made for the various hospitals where patient recruitment took place; and 3) the short period of data collection, which might have influenced the end results.
CONCLUSION
Atopic dermatitis (AD) is an infrequent finding in adults made by dermatology consultations in the Cameroonian cities of Douala and Yaoundé. It is predominantly found in females, and can either be isolated or associated with other forms of atopy. It is also found in patients with a family history of atopy. In most cases, its clinical presentation is quite moderate, but significantly affects the quality of life (QoL) of patients with AD. Therefore, in addition to medical treatment, educational and psychological support has to be provided. Such a combination of therapies could significantly improve the long-term condition of patients with AD. Assessing the QoL of adults with AD is an important step in their treatment and follow-up care.
ACKNOWLEDGMENTS
The authors are most grateful to the directors and heads of the dermatology services of the seven hospitals—five in Yaoundé and two in Douala—where this study was conducted, and to all personnel for their kind help in the process of collecting data.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis:implications for management in children. Children. 2019;6:108.
2. Ahogo KC, Kouassi YI, Gbery IP, Azagoh KR, Yeboua KI, Kouassi KA, et al. Atopic dermatitis in children:epidemiological and clinical aspects in Côte d'Ivoire. Our Dermatol Online. 2017;8(Suppl.1):25-7.
3. Nakatsuji T, Gallo RL. The role of the skin microbiome in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:263-9.
4. Hello M, Aubert H, Bernier C, Néel A, Barbarot S. Dermatite atopique de l'adulte. Rev Med Interne. 2016;37:91-9.
5. Kanwar AJ. Adult-onset atopic dermatitis. Indian J Dermatol. 2016;61:662–3.
6. Slattery MJ, Essex MJ, Paletz EM, Vanness ER, Infante M, Rogers GM, et al. Depression, anxiety, and dermatologic quality of life in adolescents with atopic dermatitis. J Allergy Clin Immunol. 2011;128:668-71.
7. Mozaffari H, Pourpak Z, Pourseyed S, Farhoodi A, Aghamohammadi A, Movahadi M, et al. Quality of life in atopic dermatitis patients. J Microbiol Immunol Infect. 2007;40:260-4.
8. de Bruin Weller MS, Rockmann H, Knulst AC, Bruijnzeel-Koomen CAFM. Evaluation of the adult patient with atopic dermatitis. Clin Exp Allergy. 2013;43:279-91.
9. Kouotou EA, Nansseu JR, Tuekam Tuekam EG, Tatah SA, Sieleunou I, Ndjitoyap Ndam EC. Atopic dermatitis in Cameroon:quality of life and psychiatric comorbidities among affected children and adolescents. Clin Pediatr Dermatol. 2017;3:6.
10. Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis:disease burden, measurement, and treatment benefit. Am J Clin Dermatol. 2016;17:163-9.
11. Pesce G, Marcon A, Carosso A, Antonicelli L, Cazzoletti L, Ferrari M, et al. Adult eczema in Italy:prevalence and associations with environmental factors. J Eur Acad Dermatol Venereol. 2015;29:1180-7.
12. Ezzedine K, Kechichian E. Epidémiologie de la dermatite atopique. Ann Dermatol Vénéréologie. 2017;144:VS4-7.
13. Orfali RL, Shimizu MM, Takaoka R, Zaniboni MC, Ishizaki AS, Costa AA, et al. Atopic dermatitis in adults:clinical and epidemiological considerations. Rev Assoc Med Bras (1992). 2013;59:270-5.
14. Zeppa L, Bellini V, Lisi P. Atopic dermatitis in adults. Dermatitis. 2011;22:40-6
15. Yemaneberhan H, Flohr C, Lewis SA, Bekele Z, Parry E, Williams HC, et al. Prevalence and associated factors of atopic dermatitis symptoms in rural and urban Ethiopia. Clin Exp Allergy. 2004;34:779-85.
16. Xu F, Yan S, Li F, Cai M, Chai W, Wu M, et al. Prevalence of childhood atopic dermatitis:an urban and rural community-based study in Shanghai, China. PLoS One. 2012;7:e36174.
17. Domínguez O, Plaza AM, Alvaro M. Relationship between atopic dermatitis and food allergy. Curr Pediatr Rev. 2020;16:115-22.
18. Benhamou AH, Eigenmann PA. Dermatite atopique et allergies alimentaires. Rev Med Suisse. 2007;3:1038-43.
19. Hello M, Aubert H, Bernier C, Néel A, Barbarot S. Dermatite atopique de l'adulte. Rev Mdd Interne. 2016;37:91-9.
20. Pefura Yone EW, Jeddi Z, Kouotou EA, Delimi B, El Gueddari Y, Karkar R, et ai. Etat des lieux de la dermatite atopique de l'enfant et de l'adulte en Afrique sub-saharienne et au Maghreb. Rev Fr Allergol. 2020;60:297-9.
21. Cheok S, Yee F, Song Ma JY, Leow R, Ho MSL, Yew YW, et al. Prevalence and descriptive epidemiology of atopic dermatitis and its impact on quality of life in Singapore. Br J Dermatol. 2018;178:276-7.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-3879-2659 http://orcid.org/0000-0003-3879-2659
|



Comments are closed.