Purpuric rash in a girl with a positive SARS-CoV-2 IgA result
Piotr Brzezinski 1,2, Uwe Wollina3, Anca Chiriac4,5,6
1,2, Uwe Wollina3, Anca Chiriac4,5,6
1 Department of Physiotherapy and Medical Emergency, Faculty of Health Sciences, Pomeranian Academy, Slupsk, Poland; 2 Department of Dermatology, Provincial Specialist Hospital in Slupsk, Ustka, Poland; 3Department of Dermatology and Allergology, Städtisches Klinikum Dresden, Academic Teaching Hospital of the Technical University of Dresden, Dresden, Germany; 4Department of Dermatology, Apollonia University, Iasi, Romania; 5Division of Dermatology, Nicolina Medical Center, Iasi, Romania; 6”P. Poni” Institute of Macromolecular Chemistry, Romanian Academy, Iasi, Romania
Corresponding author: Piotr Brzezinski, MD PhD
Submission: 16.06.2020; Acceptance: 22.08.2020
DOI: 10.7241/ourd.2020S2.4
Cite this article: Brzezinski P, Chiriac A, Wollina U. Purpuric rash in a girl with a positive SARS-CoV-2 IgA result. Our Dermatol Online. 2020;11(Supp. 2):13-14
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
In December 2019, China reported the first group of pneumonia cases associated with a new coronavirus, SARS-CoV-2, which now has become a pandemic.
Herein, we report a case of purpuric rash in a girl with only a positive SARS-CoV-2 IgA result, and a negative SARS-CoV-2 IgG result (negative nasopharyngeal swab).
A 5-year-old female presents herself with a purpuric rash on the trunk and, to a lesser extent, on the upper and lower limbs (Fig. 1). The cutaneous lesions observed are petechiae and macules (Fig. 2). The skin lesions persisted for over one week but with no symptoms reported.
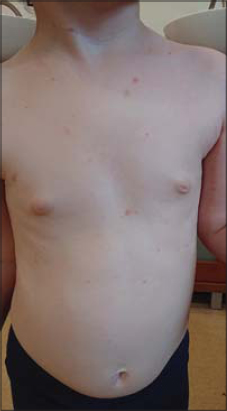 |
Figure 1: Purpuric rash in a 5-year-old female. |
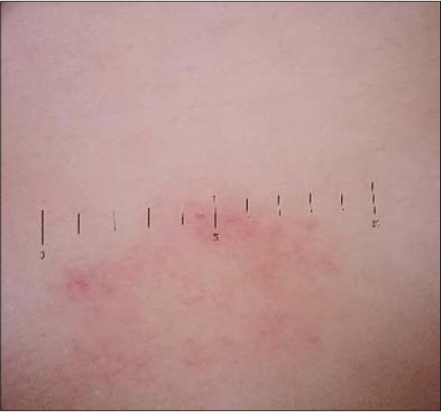 |
Figure 2: Lesion in a dermoscopy image. |
Given the high suspicion of a viral infection with SARS-CoV-19, as well as a recent trip abroad, the patient was screened for COVID-19.
The results of real-time reverse transcription polymerase chain reaction from a nasopharyngeal swab against SARS-CoV-2 were negative. On the other hand, the patient had IgA antibodies against SARS-CoV-2, but IgG antibodies were not detected (EUROIMMUN Anti-SARS-CoV-2 ELISA).
Descriptions of petechiae resembling dengue fever appear in the literature [1]. However, the lesions we observed were more concentrated and limited and without fever.
It has been suggested that a rash with petechiae or a purpuric rash seems to be a symptom of milder COVID-19. Differential diagnosis includes drug-induced rash and rashes due to other viral diseases.
Wollina described Schamberg-like purpuric eruptions and tonsillitis in a 13-year-old female [2]. Similarly to our patient, she was in good health, with no fever or malaise.
A German study evaluating three fully automated SARS-CoV-2 antibody assays yielded discordant results in three COVID-19 patients, whereas, in one COVID-19 patient, none of the investigated assays detected antibodies [3]. These assays were highly specific and sensitive in detecting SARS-CoV-2 antibodies in samples obtained 14 days or more after PCR-confirmed infection.
Authors of an international group obtained comparable results [4], emphasizing that the tests are likely to have a useful role in detecting previous SARS-CoV-2 infections if employed 15 days or more after the onset of symptoms. They may, however, carry a risk of error.
In conclusion, keeping COVID-19 in the differential diagnosis of rash is an important clue, as, otherwise, patients may be misdiagnosed.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
REFERENCES
1. Wollina U, Karada?AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients:A review [published online ahead of print, 2020 May 10]. Dermatol Ther. 2020;e13549.
2. Wollina U. Schamberg-like purpuric eruptions and tonsillitis in mild COVID-19 [published online ahead of print, 2020 Jun 4]. Dermatol Ther. 2020;e13766.
3. Hörber S, Soldo J, Relker L, Jürgens S, Guther J, Peter S, et al. Evaluation of three fully-automated SARS-CoV-2 antibody assays [published online ahead of print, 2020 Aug 4]. Clin Chem Lab Med. 2020;/j/cclm.ahead-of-print/cclm-2020-0975/cclm-2020-0975.xml.
4. Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6:CD013652.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-6817-606X http://orcid.org/0000-0001-6817-606X http://orcid.org/0000-0001-5933-2913 http://orcid.org/0000-0001-5933-2913 http://orcid.org/0000-0002-0989-4931 http://orcid.org/0000-0002-0989-4931 |



Comments are closed.