Evaluation of the response of pyogenic granuloma to 0.5% topical timolol solution
Ihsan Ali Al-Turfy, Sarraa Fawaz Najim
Center of Dermatology and Venereology, Medical City, Baghdad, Iraq
Corresponding author: Dr. Sarraa Fawaz Najim
Submission: 14.03.2020; Acceptance: 15.05.2020
DOI: 10.7241/ourd.20204.4
Cite this article: Al-Turfy IA, Najim SF. Evaluation of the response of pyogenic granuloma to 0.5% topical timolol solution. Our Dermatol Online. 2020;11(4):351-356.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Pyogenic granuloma (PG) is a benign reactive vascular proliferation of the skin or mucous membranes that is classically treated surgically. Numerous case reports and case series have described successful treatment of PG and other vascular tumors with β-blockers.
Aim: The aim is to evaluate the benefit of 0.5% topical timolol in the treatment of PG and the possible factors that may affect its response to treatment.
Materials and Methods: This is a single-arm, open-label, prospective, interventional, therapeutic trial, conducted at the Center of Dermatology and Venereology in Baghdad Medical City, Baghdad, Iraq, from December 2017 through September 2019. Patients diagnosed clinically with PG were treated topically with 0.5% timolol maleate ophthalmic solution twice daily under occlusion and monitored every two weeks for response. Clinical response was evaluated based on the difference in the size of lesions after treatment, and was classified into complete response, partial response, and no response.
Results: Thirty patients were enrolled in the study. The female-to-male ratio was 1.7:1 and the adult-to-child ratio was 2:1. The overall duration of treatment with topical timolol ranged from three days to twelve weeks with a mean and SD of 4.5 ± 2.9 weeks. At the end of the study, there was a statistically significant reduction in mean tumor size (p < 0.05). Eleven patients (36.7%) achieved complete resolution with an average duration of five weeks of treatment; seven patients (23.3%) achieved partial resolution (overall response was 60%); while twelve patients (40%) displayed no response to treatment.
Conclusion: 0.5% topical timolol maleate is a safe alternative treatment option for PG regardless of the patient’s sex and age, the size of the tumor, and the duration of the disease.
Key words: Pyogenic Granuloma, Beta-blockers, Topical Timolol solution
INTRODUCTION
Pyogenic granuloma (PG), also known as lobular capillary hemangioma, is a benign vascular tumor that appears on the skin and mucous membranes. PG can occur spontaneously, in sites of injury, or within capillary malformations [1]. It is usually painless unless associated with a secondary infection. Its aesthetically displeasing appearance and its tendency to produce recurrent profuse bleeding upon minor traumas often bring these lesions into notice [2].
Generally, PG lesions do not show a tendency for spontaneous resolution while recurrence is commonly seen after incomplete removal [2]. Exceptions include drug-induced PG, scalded PG, and granuloma gravidarum, which have been reported to resolve spontaneously after the resolution of the inciting factor [3–5].
Treatment modalities are mainly interventional and include curettage and cautery, surgical excision, chemical cauterization, laser removal, and sclerotherapy. Usually, these modalities require anesthesia and are complicated by intraoperative bleeding, postoperative pain, and scarring, as well as by a high recurrence rate (7.7% to 43.5%) [2,6,7].
In avoidance of these issues, topical treatment with imiquimod [8] and different β-blockers have been tried with variable success in different reports. Oral propranolol was reported to be effective in the treatment of recurrent PG, multifocal congenital PG, and scalded PG [4,9,10]. Topical propranolol was also used in 1% and 4% concentrations and was proved to be effective in most cases [11–13].
Malik and Murphy (2014) successfully treated a teenager a PG on the finger with 0.5% timolol ophthalmic gel [14]. Wine Lee et al. (2014) reached similar results with topical 0.5–2% timolol applied 2–3 times daily for 12–24 weeks or until the resolution of lesions [9]. Khorsand et al. (2015) reported successful treatment of a 5-month-old child with a PG on the cheek with a 0.5% topical timolol gel for 24 weeks, without recurrence [15]. Gupta et al. (2016) reported a case series of ten patients with PG treated with 0.5% timolol maleate ophthalmic solution applied four times a day with variable responses but no reported side effects [16]. Yet, few clinical trials have evaluated the degree of effectiveness of topical β-blockers in the treatment of PG in different age groups and the factors that may affect the response of PG to topical β-blockers.
MATERIALS AND METHODS
This study is a single-arm, open-label, prospective, interventional, therapeutic trial, conducted at the Center of Dermatology and Venereology in Baghdad Medical City, Baghdad, Iraq, from December 2017 through September 2019. Ethical approval was granted by the Scientific Council of Dermatology and Venereology of the Iraqi Board for Medical Specializations.
Thirty patients clinically diagnosed with a pyogenic granuloma of any size or duration, regardless of age and sex, participated in the study.
Exclusion Criteria
Excluded from the study were:
- patients who had been using medications known to induce pyogenic granuloma (e.g. oral contraceptives, retinoids, and indinavir) were excluded from the study to avoid misleading results;
- pregnant women;
- lesions preceded by scald injury;
- lesions with diagnostic uncertainty;
- patients with a contraindication to β-blocker medications (asthma, severe chronic obstructive pulmonary disease, bradycardia, heart block, cardiac failure, and hypersensitivity to β-blockers).
Information regarding the nature of the disease, treatment options, the nature of the study, and the medication used was conveyed to each patient and an informed consent was taken from each patient or, in the case of children, their relative.
The medical history taken included the duration of PG, precipitating factors, symptoms, previous therapeutic interventions, recurrence after a previous intervention, underlying medical illness, and current medications. The sites and sizes of lesions were recorded. The diameter of the largest lesion was measured with a tape measure on presentation and on each subsequent visit.
Patients were given timolol maleate ophthalmic solution in a 0.5% concentration (Apimol 0.5%, Amman Pharmaceutical Industries Co., Jordan) and were instructed to apply one to three drops—with a drop containing 0.25 mg of timolol maleate—spread it on the surface of the PG twice daily, cover the lesion with a bandage to facilitate absorption and avoid trauma as much as possible. Patients were instructed to return every two weeks for assessment.
The intended treatment duration was until the resolution of the PG. If no response was observed after four weeks of treatment or a worsening, such as an increase in size or bleeding, occurred despite treatment, topical timolol was discontinued and patients were referred for surgical intervention.
On follow-up visits, the patients were asked about bleeding, pain, and any other side effects observed. Measurements and color changes were noted as guides of early response, and photographs of lesions were taken at baseline and on each follow-up visit to facilitate comparison, maintaining the same distance, position, and lighting.
Clinical response to the topical timolol therapy was evaluated depending on the difference in size between the first and last visit and identified accordingly as complete response, partial response, or no response.
- No response: no change in size or an increase in size despite continuous treatment.
- Partial response: a decrease in size in response to therapy, but no complete clearance.
- Complete response: complete resolution of lesions. Patients with complete resolution were followed up for a minimum of four weeks up to a maximum of twelve weeks.
Data organization and statistics were performed using Microsoft Office 2013 Excel. A statistically significant result was at a p of less than 0.05.
RESULTS
Thirty patients participated in the study, 19 (63.3%) females and 11 (36.7%) males, giving a female-to-male ratio of 1.7:1. Their age ranged from 6 to 55 years with a mean and SD of 28.5 ± 16.2 years. Ten (33.3%) patients were children (<18 years old) and 20 (66.7%) were adults (≥18 years old).
Patient characteristics regarding precipitating factors, presenting symptoms, and previous interventions are elucidated in Table 1.
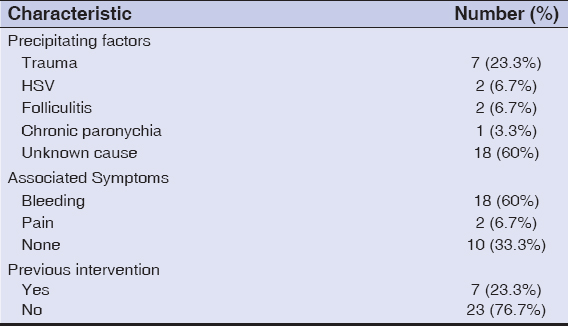 |
Table 1: Distribution of patients according to precipitating factors, associated symptoms, and a history of previous interventions. |
The duration of the disease ranged from one week to two years with a median duration of four weeks before presentation.
Nineteen lesions (63.3%) were located on the head and neck area, ten lesions (33.3%) on the extremities—mostly on one of the fingers—and one (3.3%) on the trunk.
The total duration of treatment with topical timolol ranged from three days to twelve weeks with a mean and SD of 4.5 ± 2.9 weeks.
Table 2 shows the sizes of lesions at presentation and following the 0.5% topical timolol therapy. There was a statistically significant decrease in size after treatment with timolol solution (p = 0.004).
 |
Table 2: The size of PG before and after treatment with topical timolol. |
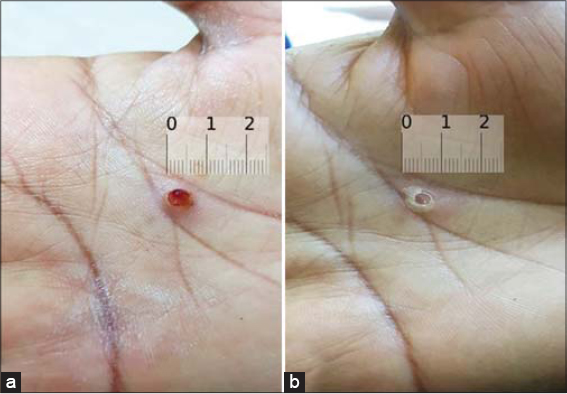
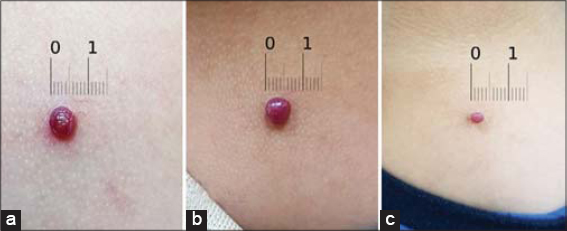
Eighteen patients (60%) displayed a measurable clinical response within three days to four weeks of treatment with 0.5% timolol, among which eleven (36.7% of all patients) achieved complete resolution (Figs. 1 and 2) with an average duration of five weeks of treatment, ranging from three days to twelve weeks, and showed no recurrence after a minimum of four weeks of follow-up. The earliest response was observed in a nine-year-old boy with a PG on the face that disappeared completely three days after starting treatment (Fig. 1). The other seven patients (23.3% of all patients) achieved only partial resolution (Fig. 3). Surgical removal was offered to these patients.
Twelve patients (40%) showed no response to treatment, among which seven achieved no change in size after four weeks of continuous application of 0.5% topical timolol twice daily. The remaining five patients experienced an increase in lesion size despite the application of topical timolol twice daily. Electrocautery was performed for these patients.
No local or systemic side effects were recorded in this trial apart from mild itching reported by one patient.
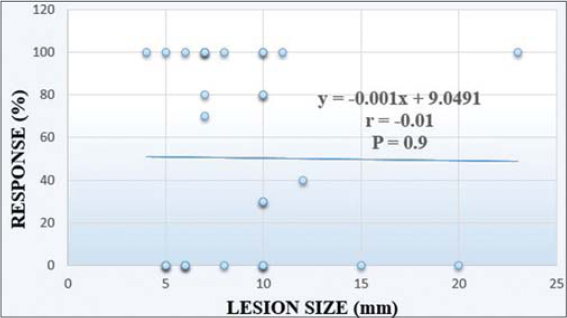
There was no statistically significant correlation between a patient’s sex or age and the response to treatment, as shown in Tables 3 and 4, respectively. Similarly, there was no statistically significant correlation between the response to treatment with the duration of a lesion (Fig. 4) or its size (Fig. 5) at presentation.
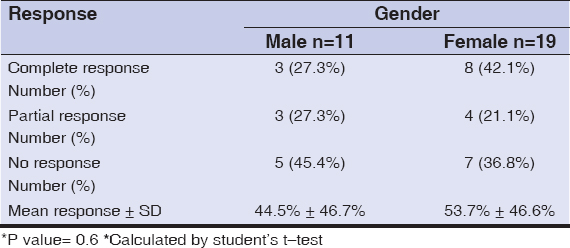 |
Table 3: The clinical response of PG to topical timolol according to sex. |
 |
Table 4: The clinical response of PG to topical timolol according to the patient’s age. |
DISCUSSION
A significant number of case reports and case series have reported the use of the topical β-blockers propranolol and timolol for the treatment of PG, but few studies have evaluated the effectiveness of these topical preparations or compared the effectiveness of different preparations and their concentrations.
In this study, the response of PG to topical timolol, a nonselective β-blocker, was evaluated in different age groups.
A topical ophthalmic 0.5% timolol preparation was chosen due to its high potency, which is about eight to ten times greater than that of propranolol [17], as well as its low side-effect profile [18–20], easy availability, and insignificant cost.
Thirty patients participated in the study. Sixty percent of them responded to topical timolol. The first response was three days after starting treatment. A complete response was seen in 36.7% of patients, a partial response in 23.3%, and no response in 40%. The mean size of PG decreased significantly after the timolol treatment.
This variation in the response of PG to topical timolol may be explained by the weak expression of β-adrenergic receptors on the surface of PG, as reported by Chisholm et al. (2012) in 50% of lesions, in contrast to the uniform strong expression of β-receptors on the surface of infantile hemangioma [21].
Chisholm, however, considered only two PG lesions, hence it seems that more research is necessary to confirm these results.
Since topical timolol is applied by the patient at home, varying degrees of compliance and adherence to treatment might be another factor explaining the variability in response.
The clinical responses in our study were comparable to those reported by Gupta et al. (2016), who found complete resolution in 40% of patients (compared to 36.67% in our study), partial resolution in 30% (compared to 23.33% in our study) and no response in 30% (compared to 40% in our study) after the application of ophthalmic 0.5% timolol maleate solution four times daily. In a study by Gupta et al. (2016), the time needed for a complete response was three to twenty-four days [16], whereas, in our study, it ranged between three days and twelve weeks. The difference in the time needed to achieve a complete response may be explained by the application topical timolol four times a day in the study by Gupta et al.
Gupta et al. could not draw a conclusion about the relationship between the response to treatment and the duration of a lesion due to a small sample size. Our study concluded that there was no statistically significant correlation between the duration of a lesion before treatment and its response to treatment.
A great number of researchers have reported higher success rates with topical propranolol than with topical timolol: Neri et al. (2018) used 1% propranolol ointment under occlusion to treat pediatric PG. Twenty-two patients received treatment, of which 59% achieved complete resolution in a mean of 9.5 weeks (compared to 36.6% of those with a mean of five weeks in our study), 18% had partial resolution after 1% propranolol ointment (compared to 23.3% in our study), and 23% showed no response to treatment (compared to 40% in our study) [11].
A higher concentration of propranolol was evaluated by Mashiah et al. (2019) in a retrospective study. Eighteen pediatric patients treated with a 4% propranolol gel twice daily without occlusion were enrolled. Eleven lesions (61.1%) resolved completely by the end of the treatment (compared to 36.6% of those treated with timolol in our study); two lesions (11.1%) almost resolved; and five (27.7%) underwent curettage [12].
The better response of PG to propranolol may be explained by the difference in age in the study population, as both of the propranolol studies were restricted to a pediatric-age group, whereas our study included different age groups. It must be noted that, in our study, the response rate in children was higher than in adults, but there was no statistically significant relationship between the age of the patient and the response to treatment.
The higher response rate in children may be due to the increased absorption through the thinner skin in children and, perhaps, also due to the higher commitment of parents to treat their children.
CONCLUSION
0.5% topical timolol is a safe alternative treatment for PG particularly if the surgical approach is difficult or contraindicated or not preferred by the patient, as in small children, elderly patients with comorbid illnesses, in cosmetically-sensitive areas such as the face, nails, and lips, and in PGs that recurred after a previous surgical intervention. However, it is not recommended to continue treatment with topical timolol if no response is achieved after four weeks.
Patients of all ages and sexes are candidates for this modality of treatment, regardless of the size or duration of their PG.
Further studies using a combination of oral and topical β-blockers, especially for large, unresponsive lesions are recommended in search of more complete and definitive results.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Wollina U, Langner D, França K, Gianfaldoni S, Lotti T, Tchernev G. Pyogenic granuloma –a common benign vascular tumor with variable clinical presentation:new findings and treatment options. Open Access Maced J Med Sci. 2017;5:423–6.
2. Calonje E. Soft-tissue tumours and tumour-like conditions.|eIn:Rook A, Barker J, Bleiker T, Chalmers R, Creamer D, Griffiths C. eds. Rook’s textbook of dermatology. 9th ed. Vol. 4. Chichester, West Sussex (UK):Wiley Blackwell;2016. 137.26-8.
3. James W, Elston D, Treat J, Rosenbach M, Neuhaus I. Andrews’Diseases of the Skin. 13th ed. Elsevier;2019. ch. 28:Dermal and Subcutaneous Tumors;p.590.
4. Sharquie KE, Noaimi AA, Radhi SK. Burn Hemangioma (BH) (scalded pyogenic granuloma) versus infantile hemangioma:report of six cases of bh and its effective therapy with oral propranolol. J Cosmet Dermatol Sci Appl. 2017;07:229–44.
5. Cardoso JA, Spanemberg JC, Cherubini K, Figueiredo MAZ de, Salum FG. Oral granuloma gravidarum:a retrospective study of 41 cases in Southern Brazil. J Appl Oral Sci. 2013;21:215–8.
6. Koo MG, Lee SH, Han SE. Pyogenic granuloma:a retrospective analysis of cases treated over a 10-year. Arch Craniofacial Surg. 2017;18:16–20.
7. Bansal N, Belgaumkar V, Chavan R, Doshi B. Successful treatment of pyogenic granuloma with sclerotherapy. Indian J Drugs Dermatol. 2019;5:30-3.
8. Badavanis G, Pasmatzi E, Monastirli A, Tsambaos D. Successful Treatment of suborbital pyogenic granuloma with topical imiquimod. Case Rep in Clin Med. 2018;7:1-6.
9. Wine Lee L, Goff KL, Lam JM, Low DW, Yan AC, Castelo-Soccio L. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonists. Pediatr Dermatol. 2014;31:203–7.
10. Alharbi M, Eber AE, Perper M, ALFalah M, Al-Khenaizan S, Alomair IA, et al. Multifocal congenital pyogenic granuloma successfully treated with oral propranolol. Pediatr Dermatol. 2019;36:41–3.
11. Neri I, Baraldi C, Balestri R, Piraccini BM, Patrizi A. Topical 1% propranolol ointment with occlusion in treatment of pyogenic granulomas:An open-label study in 22 children. Pediatr Dermatol. 2018;35:117–20.
12. Mashiah J, Hadj-Rabia S, Slodownik D, Harel A, Sprecher E, Kutz A. Effectiveness of topical propranolol 4% gel in the treatment of pyogenic granuloma in children. J Dermatol. 2019;46:245–8.
13. Piraccini BM, Alessandrini A, Dika E, Starace M, Patrizi A, Neri I. Topical propranolol 1% cream for pyogenic granulomas of the nail:open-label study in 10 patients. J Eur Acad Dermatol Venereol. 2016;30:901–2.
14. Malik M, Murphy R. A pyogenic granuloma treated with topical timolol. Br J Dermatol. 2014;171:1537–8.
15. Khorsand K, Maier M, Brandling-Bennett HA. Pyogenic granuloma in a 5-month-old treated with topical timolol. Pediatr Dermatol. 2015;32:150–1.
16. Gupta D, Singh N, Thappa DM. Is timolol an effective treatment for pyogenic granuloma?Int J Dermatol. 2016;55:592–5.
17. MacArthur KM, Puttgen K. Vascular tumors.|eIn:Kang S, Amagai M, Bruckner A, Enk A, Margolis D, McMichael A, et al.eds. Fitzpatrick’s Dermatology. 9th ed. Mc Graw Hill Education;2019. p.2089-90.
18. Gallegos AC, Davis MJ, Tchanque-Fossuo CN, West K, Eisentrout-Melton A, Peavy TR, et al. Absorption and Safety of Topically Applied Timolol for Treatment of Chronic Cutaneous Wounds. Adv Wound Care (New Rochelle). 2019;8:538-45.
19. Duell K, McNab S. Propranolol and topical timolol for infantile haemangiomas of the skin. J Paediatr Child Health. 2020;56:480-2.
20. Puri N. A study on the treatment of infantile hemangiomas with topical timolol. Indian J Paediatr Dermatol. 2020;21:116-8.
21. Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-McKenney AE. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446–51.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|





Comments are closed.