Gingival mucormycosis: case report and literature review
Alexandro Bonifaz 1, Ana Gabriela Fuentes-Nava1, Andrés Tirado-Sánchez1, Juan J. Kassack2, Juan D. Vega-Muñoz2, Rogelio Treviño-Rangel3, Gloria M. González2
1, Ana Gabriela Fuentes-Nava1, Andrés Tirado-Sánchez1, Juan J. Kassack2, Juan D. Vega-Muñoz2, Rogelio Treviño-Rangel3, Gloria M. González2
1Dermatology Service, and Mycology Department. Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico, 2Hematology Service. Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico, 3Department of Microbiology, School of Medicine, Universidad Autónoma de Nuevo León, Mexico
Corresponding author: Prof. Alexandro Bonifaz
Submission: 25.06.2020; Acceptance: 01.10.2020
DOI: 10.7241/ourd.20204.15
Cite this article: Bonifaz A, Gabriela Fuentes-Nava A, Tirado-Sánchez A, Kassack JJ, Vega-Muñoz JD, Treviño-Rangel R, González GM. Gingival mucormycosis: case report and literature review. Our Dermatol Online. 2020;11(4):389-392.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Mucormycosis is a rapidly progressive fungal infection characterized by endothelium invasion and the development of thrombi in blood vessels resulting in necrosis. Early diagnosis is crucial for effective treatment. Oral mucormycosis is an uncommon and possibly an underestimated disease. Herein, we present the case of a 31-year-old male previously diagnosed with refractory L2 acute lymphoblastic leukemia (ALL) who suffered gingival mucormycosis due to Rhizopus arrhizus. Empirical treatment with amphotericin B deoxycholate (ABD) was prescribed with clinical and mycological healing on day 17. Unfortunately, the patient had an unfavorable outcome because of the ALL and died 49 days after the admission due to multiple organ failure.
Key words: Mucormycosis, Hematological malignancies, Platelet count, Rhizopus arrhizus
INTRODUCTION
Mucormycosis is a fungal infection with a high mortality rate (>50%) caused by the Mucorales often occurring in immunosuppressed patients, as with diabetes mellitus, hematopoietic stem cell transplants, or associated hematological malignancy. Mucormycosis usually develops as an acute infection and presents itself in rhinocerebral, pulmonary, gastrointestinal, cutaneous, and disseminated clinical types. Oral mucormycosis is rarely seen in clinical practice and reported cases are scarce [1–3]. Herein, we present the case of a male patient with L2 acute lymphoblastic leukemia (ALL) with gingival mucormycosis, as well as a short review of the literature.
CASE REPORT
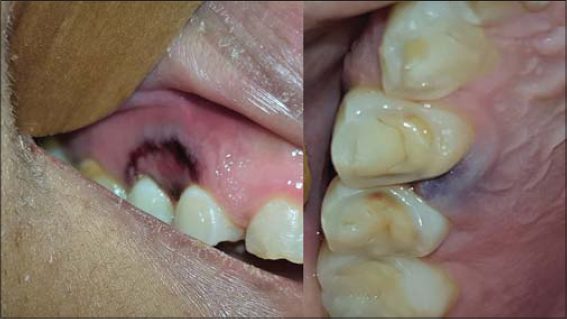
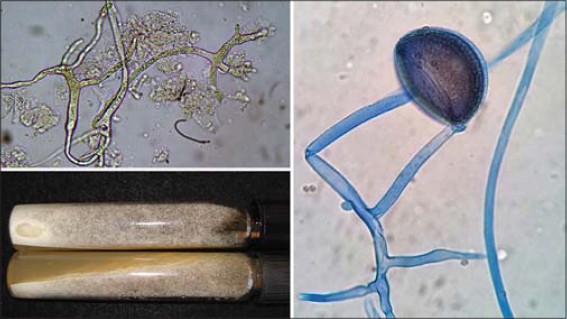
A 31-year-old male, previously diagnosed with L2 acute lymphoblastic leukemia (ALL) since 2016, was admitted to our hospital in April 2019 because of the fourth disease relapse. On admission, the patient had pancytopenia, severe leukopenia (2,100 cells/μL), thrombocytopenia (99,000 cells/μL), and anemia (8.8 g/dL). Treatment was started with a Hyper-CVAD regimen (hyperfractionated cyclophosphamide doses, vincristine, doxorubicin, and dexamethasone) but without clinical improvement. The leukopenia persisted through day 2 and a progressive decrease in platelet levels was observed (3,000 cells/μL). During the hospital stay, several necrotic ulcers developed at the first and second premolars of the right maxilla in the gingival region, as well as an edematous plaque over the hard palate (Fig. 1). A direct microscopic examination revealed broad, dichotomic, coenocytic, and hyaline hyphae. Sabouraud dextrose agar media yielded hairy, cottony, grayish-white colonies with broad non-septate hyphae, sporangiophores, rhizoids, sporangia, and sporangiospores visualized. The fungus was mycologically identified as Rhizopus arrhizus (formerly R. oryzae) and confirmed by molecular biology according to the following regions: ITS: 99/99.2 (NCBI), 95.67/100 (ISHAM-ITS), 96.64/100 (MycoBank); and D1/D2: 99/99.1 (NCBI), 95.26/99.53 (MycoBank). Hence, we reached a diagnosis of gingival (oral) mucormycosis caused by Rhizopus arrhizus. A biopsy was not taken due to severe thrombocytopenia (Fig. 2). A CT scan of the head failed to show evidence of bone involvement. Surgical debridement was not performed because of the patient’s condition. Amphotericin B deoxycholate (ABD) was started at 1 mg/kg/day with clinical and mycological healing on day 17. Unfortunately, the patient’s condition worsened with disseminated intravascular coagulation and the patient died on day 49.
DISCUSSION
Mucormycosis is an invasive fungal infection characterized by the rapid growth of filamentous fungi, leading to thrombosis and tissue necrosis [1,4]. Rhizopus arrhizus is the most common etiologic agent, followed by Mucor circinelloides and Lichtheimia corymbifera, which together account for around 70% of all infections [1,4–7]. In 2005, Roden et al. conducted a review of 929 cases [4]. The mean age was 38.8 years with a prevalence of males (65%). DM was the most common underlying condition. One hundred fifty-four patients presented with a malignant neoplasm, among which 147 (95%) were hematological.
The Mucorales have the ability to cross physical barriers, finding innate immune cells: macrophages, neutrophils, and dendritic cells. Angioinvasion is a distinctive process in mucormycosis. Endothelium breakage causes thrombosis and tissue necrosis and allows for hematogenous dissemination. The R. arrhizus adheres directly to the endothelial cells and impels lesions through its internalization. The entrance to the endothelial cells is arbitrated by the endothelial cell surface receptor GRP78, which significantly increases acidosis and hyperglycemic conditions [5]. Patients with neutropenia have more extensive angioinvasion [8,9]. After crossing the endothelial tissue, Mucorales find platelets, recently identified as key effectors of the innate immune system, as they bear antimicrobial properties mediated by the release of platelet antimicrobial peptides such as platelet factor 4 (PF-4), as well as chemotactic properties mediated by the release of cytokines such as IL-1b. In vitro studies have shown that the platelets adhere to both spores and hyphae and significantly inhibit fungal germination and cause direct damage to hyphae [2,5].
Oral mucormycosis usually develops after the transpalatal extension of rhinocerebral infection. Cases located in the periodontal tissue (gingival and alveolar bone) are rare [8–10]. In general, we have had experience of palatal ulcer formation in up to a third of our patients, but gingival presetting is extraordinary and must be kept in mind in the oral forms of mucormycosis [10]. Table 1 shows a comparison of the characteristics of the reported cases, including this case.
In our search through the English-language literature, we found merely ten cases of periodontal mucormycosis: nine in patients with hematological malignancies and one in a diabetic patient. All patients underwent extension studies and the diagnosis of aspergillosis was ruled out. All cases, including this case, had active hematological malignancies. All patients had neutropenia (<500 cells/μL) and four patients with hematological malignancy had associated thrombocytopenia. Clinical manifestations were similar: the patients complained of gingival pain while seven patients had a fever. Physical examinations revealed bluish, grayish, or whitish edematous plaques as well as necrotic changes. All patients received amphotericin B as a first-line treatment and three patients underwent surgical debridement. Seven of the eleven cases successfully completed the antifungal treatment and two patients died of pneumonia whereas our patient died from multiple organ failure. In one patient, the evolution was unknown [11]. The diabetic patient received treatment with amphotericin B and surgical debridement with favorable results [12]. Unlike rhinocerebral mucormycosis, the gingival type was observed without central nervous system involvement and with slower progression [1,8,13].
Mucormycosis is diagnosed by direct examination with Calcofluor, Fungifluor, or Blankofluor. Calcofluor analysis is not performed in daily practice due to the need for fluorescence microscopy [9]. Laboratory diagnosis is necessary, although bearing low sensitivity. The Mucorales grow rapidly to 37°C (98.6°F) in selective and nonselective media. The diameter of coenocytic (non-septate) or pauci-septate hyphae ranges from 6 to 25 μm. The branch angle is variable and includes bifurcations with an angle of ≥90° [8]. Histopathological examination makes it possible to distinguish between Aspergillus hyphae and Mucorales hyphae, defining the treatment. Mucormycosis is characterized by necrosis and vascular and perineural invasion [1]. No standardized tests are available for detecting Mucorales-specific antigens. The results of negative serum galactomannan and bronchoalveolar lavage tests support the diagnosis of mucormycosis [14]. (1,3)-β-D-glucan is a common part of the cell wall of a wide variety of fungi, but not the Mucorales [1].
Amphotericin B deoxycholate is one of the drugs approved for treating mucormycosis. However, given the associated toxicities, it is often replaced by lipid formulas. Early treatment with amphotericin B and the surgical debridement of the infected areas is the treatment of choice [1,14]. Posaconazole has shown significant clinical efficacy against the Mucorales [8,15]. Isavuconazole is a novel azole showing in vitro and in vivo activity against the Mucorales when compared with posaconazole [1,15]. On the other hand, fluconazole, voriconazole, echinocandins, and flucytosine show no in vitro activity against the Mucorales [1].
In hematologic patients, it is suggested to continue treatment until complete remission is achieved proved by imaging studies and reversing risk factors [1]. Alveolar osteonecrosis presents difficulty in surgical treatment since extensive debridement of the maxillary bones may trigger functional complications. In addition, the risks and benefits of surgical treatment should be carefully weighed in patients with neutropenia and thrombocytopenia [8]. During severe immunosuppression, primary prophylaxis with posaconazole has been recommended specifically for the prevention of mucormycosis, but a lack of controlled studies persists [1].
CONCLUSION
This was the case of a patient with L2 ALL displaying rare clinical primary gingival mucormycosis. The patient had an unfavorable outcome because, despite treatment, the ALL activity persisted, together with severe neutropenia and thrombocytopenia. The patient died due to multiple organ failure. In hematologic patients, prognosis usually depends on the response to treatment of hematologic neoplasia.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis:an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405-21.
2. Cheong HS, Kim SY, Ki HK, Kim JY, Lee MH. Oral mucormycosis in patients with haematologic malignancies in a bone marrow transplant unit. Mycoses. 2017;60:836-41.
3. Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico:epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;1;56:29-43.
4. Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis:a review of 929 reported cases. Clin Infect Dis. 2005;41:634-53.
5. Ghuman H, Shepherd-Roberts A, Watson S, Zuidscherwoude M, Watson SP, Voelz K. Mucor circinelloides induces platelet aggregation through integrin ?IIb?3 and Fc?RIIA. Platelets. 2019;30:256-63.
6. Cohen A, Shoukair FL, Korem M, Shaulov A, Casap N. Successful mandibular mucormycosis treatment in the severely neutropenic patient. J Oral Maxillofac Surg. 2019;77:1209.e1-1209.e12.
7. Auluck A. Maxillary necrosis by mucormycosis. a case report and literature review. Med Oral Patol Oral Cir Bucal. 2007;12:E360-4.
8. McDermott NE, Barrett J, Hipp J, Merino MJ, Lee CCR, Waterman P, et al. Successful treatment of periodontal mucormycosis:report of a case and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e64-9.
9. Benites BM, Fonseca FP, Parahyba CJ, Arap SS, Novis YA, Fregnani ER. Extensive oral mucormycosis in a transplanted patient. J Craniofac Surg. 2017;28:e4-5.
10. Bonifaz A, Macias B, Paredes-Farrera F, Arias P, Ponce RM, Araiza J. Palatal zygomycosis:experience of 21 cases. Oral Dis. 2008;14:569-74.
11. Salisbury PL III, Caloss R Jr., Cruz JM, Powell BL, Cole R, Kohut RI. Mucormycosis of the mandible after dental extractions in a patient with acute myelogenous leukemia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:340-4.
12. Dogan MC, Leblebisatan G, Haytac MC, Antmen B, Surmegozler O. Oral mucormycosis in children with leukemia:report of 2 cases. Quintessence Int. 2007;38:515-20.
13. Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect 2006;12:181-4.
14. Takemoto K, Yamamoto Y, Kanazawa K. Comparative study of the efficacy of liposomal amphotericin B and amphotericin B deoxycholate against six species of Zygomycetes in a murine lethal infection model. J Infect Chemother. 2010;16:388-95.
15. Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR III, et al. Isavuconazole treatment for mucormycosis:a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828-37.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-2045-3317 http://orcid.org/0000-0003-2045-3317 http://orcid.org/0000-0001-9306-1619 http://orcid.org/0000-0001-9306-1619 http://orcid.org/0000-0002-4433-6556 http://orcid.org/0000-0002-4433-6556 http://orcid.org/0000-0001-6874-7176 http://orcid.org/0000-0001-6874-7176 |



Comments are closed.