Lichen simplex chronicus positive for C5b-9/MAC, IgD and C3c as a result of recurrent bacterial hair follicular unit infection
Ana Maria Abreu Velez 1, Billie L. Jackson2, Michael S. Howard1
1, Billie L. Jackson2, Michael S. Howard1
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA, 2Billie L. Jackson, M.D., Dermatologist, Macon, Georgia, USA
Corresponding author: Ana Maria Abreu Velez, M.D., Ph.D.
Submission: 30.06.2020; Acceptance: 03.10.2020
DOI: 10.7241/ourd.20204.14
Cite this article: Abreu Velez AN, Jackson BL, Howard MS. Lichen simplex chronicus positive for c5b-9/MAC, IgD and C3c as a result of recurrent bacterial hair follicular unit infection. Our Dermatol Online. 2020;11(4):385-388.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Lichen simplex chronicus (LSC) classically results from chronic scratching, and is often associated with an underlying skin condition. A 20-year-old female presented with recurrent inflammatory itchy papules and pustules, and thickened plaques for over six months duration on her arms. The patient had no clinical history of acne or rosacea. Skin biopsies demonstrated focal spongiosis and parakeratosis within the epidermis and histologic evidence of LSC. Gram positive bacteria were observed inside the sebaceous glands. DIF in the hair follicles was positive for IgD and C3c. IHC positivity with HLA-ABC and C5b-9/MAC was seen in the same areas. Our case illustrates how a chronic bacterial folliculitis may create an ongoing cycle of chronic inflammation and the case needs to be further investigate. In addition, it illustrates the importance of utilizing DIF and IHC in addition to H&E review in cases of LSC with obscure etiologies.
Key words: Hair follicle inflammation, lichen simplex chronicus, IgD, HLA-ABC, C5b-9, Gram positive
Abbreviations: Hematoxylin and eosin (H&E), immunohistochemistry (IHC), direct, immunofluorescence (DIF), basement membrane zone (BMZ), 4’,6-diamidino-2-phenylindole (DAPI), Ulex europaeus agglutinin (Ulex), lichen simplex chronicus (LSC).
INTRODUCTION
Lichen simplex chronicus (LSC) may occur after chronic rubbing, eczema, lichen planus, stress, insect bites, dry skin, atopic dermatitis, neuropathies and amyloidosis, among many other conditions [1]. It is thus important to determine the etiology of each case of LSC.
CASE REPORT
A 20 year old female consulted a dermatologist, presenting with a greater than six month history of recurrent, erythematous papules and pustules on her arms with no clinical history of acne or rosacea. In addition, she presented with circumscribed pruritic plaques on the dorsal aspect of her arms. The patient described severe itching, and frustration because many previous physicians had believed that no underlying disease was present. Before her biopsies, ampicillin, Atarax® and mupirocin ointment had been prescribed with no improvement. She then decided to consult the dermatologist. Skin biopsies were obtained for hematoxylin and eosin(H&E) review, for immunohistochemistry (IHC) and for direct immunofluorescence (DIF) studies; these were performed as previously described [2]. No tissue culture was performed from the biopsies. After receiving the diagnosis, prednisone was given by for 3 weeks and did not help; fluocinonide ointment, 0.05% then provided improvement and relief for the patient.
Skin biopsies were taken for hematoxylin and eosin (H&E) staining, direct immunofluorescence (DIF) and for immunohistochemistry (IHC); all were performed as previously described [2]. For DIF, we classified our findings as previously categorized [2], i.e., negative (-), weakly positive (+), positive (+++) and strongly positive (++++).
IHC stains were performed utilizing a Leica Bond MAX IHC automatized platform stainer (Buffalo Grove, Illinois, USA) using a Novolink™ detection with Compact Polymer™ technology. Specifically, for primary staining we utilized a bond polymer refined Red detection DS9390, an alkaline phosphatase linker polymer and fast red chromogen (red staining). For our secondary staining, we utilized bond polymer refined detection DS9800, a horseradish peroxidase linker polymer and DAB chromogen (brown staining). Positive and negative controls were consistently performed. We used anti-human monoclonal antibody to HLA-ABC antigen, clone W6/32, polyclonal rabbit anti-human IgD (code IR517), and C5B-9 (code M077) from Agilent-Dako (Carpinteria, California, USA).
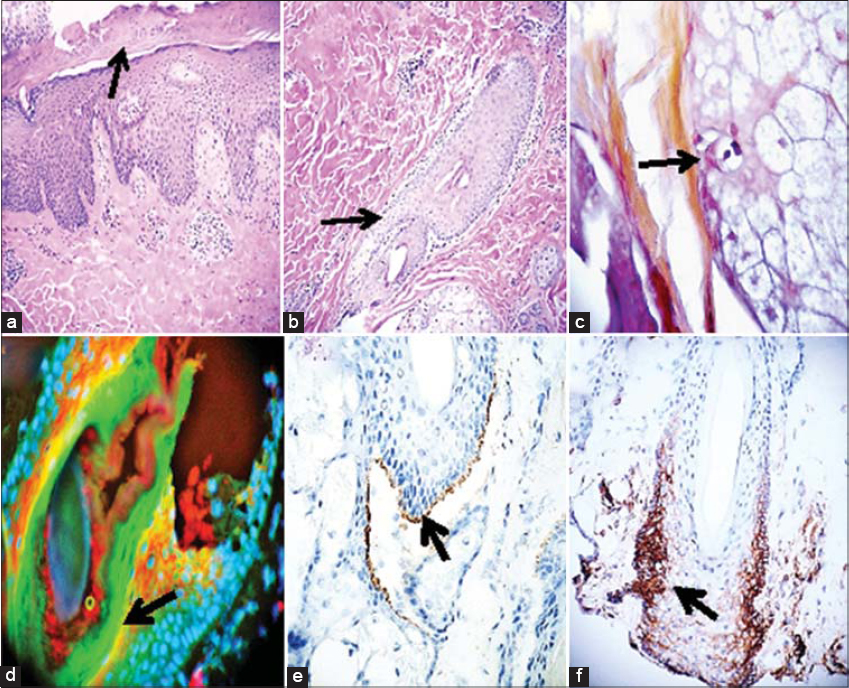
Microscopic Description of the H&E tissue sections demonstrated focal spongiosis and parakeratosis within the epidermis. Evidence of LSC was present, with no ulceration. Within the dermis, a mild, superficial, perivascular infiltrate of lymphocytes and histiocytes with occasional eosinophils and mast cells was identified around blood vessels supplying the hair follicles (Fig. 1). A Gram stain confirmed the localized presence of Gram positive coccal bacteria in some BMZ and internal areas of the hair follicles and sebaceous glands.
DIF displayed the following results: IgG (++; dotted cell junctions in epidermal corneal keratinocytes), IgD and C3c (+++, scattered positivity within sebaceous gland lobules and hair follicle isthmus areas; colocalizing with both the Gram positive staining and the perifollicular infiltrate; +++); IgE (++), positive in the epidermal keratinocytes of the corneal layer, as well as inside the sebaceous glands. Opsonized bacteria were found inside the hair follicles, in areas positive for C3c and IgD (Fig. 1).
Complement C5b-9 was as well as HLA-ABC were positive in several spots in the basement membrane zone (BMZ) of the sebaceous glands as well as the BMZ of the hair follicles where the Gram-positive bacteria were seen (Fig. 1).
DISCUSSION
Lichen simplex chronicus (LSC) is a localized, well-circumscribed area of lichenification often resulting from repeated rubbing or scratching of the skin; it often results in tremendous patient discomfort and frustration. It occurs more in skin of patients affected by atopic, seborrheic and/or contact dermatitides, as well as psoriasis [1]. We present a case of chronic hair follicle inflammation induced by Gram positive bacteria. The infection resulted in strong reactivity via IgD and other immunoglobulins and complement that prolonged the subclinical inflammation. The hair follicles as well as the pilosebaceous units can be recurrently inflamed by infectious and parasitic entities. Not only in humans, but also in animals, often antimicrobial therapy is often given in superficial bacterial folliculitis but now knowing the underlying cause. One of the most common infections are seen due to Demodex mites, Malassezia spp [3–5]. Hookworms had been also associated with folliculitis [6]. Hair restoration using some synthetic fibers can also be cause of folliculitis [7].
We were able to confirm the small foci of Gram positive bacteria because the DIF and the IHC studies helped to localize them. IgD and C3c were the most consistently positive immune markers around the bacteria as well as in the perifollicular infiltrate. Of interest, both C5b-9/MAC and HLA-ABC were very positive in the area of the folliculitis and the inflammatory infiltrate. The terminal complement component [membrane attack complex (MAC)], A.K.A C5b-9. In some cases, it has been demonstrated that bacterial killing by complement requires direct anchoring of membrane attack complex precursor C5b-7. A significant effector role of the human complement system is to straight kill Gram-negative bacteria via Membrane Attack Complex (MAC) pores. MAC pores are assembled when surface-bound convertase enzymes convert C5 into C5b, which together with C6, C7, C8 and numerous copies of C9 forms a transmembrane pore that damages the bacterial cell envelope [8]. This may explain the reactivity that we observed using the IHC C5b-9/MAC stain.
In cases like ours and/or in any suspected recurrent folliculitis or pilosebaceous unit infection, advanced techniques may be helpful. These include 1) fluorescence in situ hybridization (FISH) using broad-range bacterial and fungal probes; 2) immunofluorescence microscopy, and 3) monoclonal antibodies directed towards Gram-positive bacteria and/or against Staphylococcus spp. and Propionibacterium acnes [3–4]. Most laboratories and/or medical practitioners lack these techniques; in our case, these tests were not performed. Of interest, when using DIF we were able to identify coccal bacteria labelling with IgD, C3c and, to a lesser degree IgE inside the sebaceous glands and hair follicular units.
CONCLUSION
In cases of recurrent lichen simplex chronicus of unknown etiology, it may be helpful to utilize direct immunofluorescence and immunohistochemistry in addition to a H&E histologic review. These combined methodologies can enhance confirmation of Gram stained bacterial infections.
STATEMENT OF ETHICS
Our patient gave informed consent. Although Institutional Review Board (IRB) approval for a case report is not needed, the US Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy Rule restricts how protected health information (individually identifiable health information) on any patient may be used. Compliance with patient privacy, institutional rules, and federal regulations were followed. Specifically, no photos or illustrations that contain identifiable features are included in the case report, and the case described in the report is not so unique or unusual that it might be possible for others to identify the patient.
REFERENCES
1. Charifa A, Badri T. Lichen Simplex Chronicus. 2020 May 24. In:StatPearls [Internet]. Treasure Island (FL):StatPearls Publishing;2020 Jan.
2. Abreu-Velez AM, Upegui-Zapata YA, Valencia-Yepes CA, et al. J Cutan Pathol. 2019;46:925-29.
3. Perego R, Spada E, Martino PA, Proverbio D. Diagnostic evaluation of a point-of-care test for culture and microbial susceptibility testing in canine dermatological infections in clinical practice. Vet World. 2020;13:521-29.
4. Jahns AC, Lundskog B, Berg J, Jonsson R, McDowell A, Patrick S, et al. Microbiology of folliculitis:a histological study of 39 cases. APMIS 2014;122:25-32.
5. Lander M, Checkley AM, Walker SL. Cutaneous larva migrans presenting with folliculitis. Am J Trop Med Hyg. 2020;102:919-20.
6. Saenz Aguirre A, Martínez de Salinas Quintana A, de la Torre Gomar FJ, et al Hookworm:An Uncommon Cause of Folliculitis in Travelers. Actas Dermosifiliogr. 2020;111:275-76.
7. Vlachos C, Henning MAS, Gaitanis G, Faergemann J, Saunte DM. Critical synthesis of available data in Malassezia folliculitis and a systematic review of treatments. J Eur Acad Dermatol Venereol. 2020;34:1672-83.
8. Doorduijn DJ, Bardoel BW, Heesterbeek DAC, Ruyken M, Benn G, Parsons ES, et al. Bacterial killing by complement requires direct anchoring of membrane attack complex precursor C5b-7. PLoS Pathog. 2020;16:e100?.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0003-0430-6093 http://orcid.org/0000-0003-0430-6093 |



Comments are closed.