Lesional IgE in flares induced by drugs in a patient with autoimmune lupus erythematosus
Ana Maria Abreu-Velez 1, Bruce R. Smoller23, Michael S. Howard1
1, Bruce R. Smoller23, Michael S. Howard1
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA; 2Department of Pathology and Laboratory Medicine, New York, USA; 3Department of Dermatology, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA
Corresponding author: Ana Maria Abreu Velez, M.D., Ph.D.
Submission: 15.04.2020; Acceptance: 01.06.2020
DOI: 10.7241/ourd.20203.8
Cite this article: Abreu-Velez AM, Smoller BR, Howard MS. Lesional IgE in fl ares induced by drugs in a patient with autoimmune lupus erythematosus. Our Dermatol Online. 2020;11(3):260-263.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Flares in systemic lupus erythematosus (SLE) may be triggered by multiple factors. Here we present a case of a flare in an SLE patient triggered by drugs. A 53 year old female with SLE presented to the dermatologist with an annular, scaly, itchy rash with vesicles on her body and with malaise, fever and joint pain. The rash started after she began receiving sulfasalazine and erythromycin for a “non-healing leg ulcer”. The H&E demonstrated subcorneal blisters with neutrophils, hyperkeratosis and follicular plugging. The IHC and DIF displayed reactivity with IgE in the upper dermal vessels, and among cell junctions in the epidermis. The DIF also demonstrated staining in the blister with multiple immunoglobulins, and fibrinogen in the basement membrane. The remainder of the basement membrane zone displayed the classic lupus band. We demonstrate that the IgE deposits in skin from lupus patients with a flare occurring suddenly after receiving new medications may indicate a superimposed allergic reaction. Our case shows for the first time that IgE needs to be studied in confirmed lupus patients with a new medication associated flare.
Key words: Flares in lupus, IgE, drug induced lupus flares
Abbreviations: Systemic lupus erythematosus (SLE), hematoxylin and eosin (H&E), immunohistochemistry (IHC), basement membrane zone (BMZ) direct and indirect immunofluorescence (DIF, IIF), myeloperoxidase (MPO).
INTRODUCTION
Lupus flares may occur abruptly and without clear cause, and patients frequently notice a return of the symptoms they previously experienced. Drug-induced lupus flares are different from classic lupus erythematosus [1].
Drug-induced lupus is different from lupus flares in patients with known autoimmune lupus erythematous [2–7]. To our surprise, we searched the MeSH headings (previously similar to key words), and also specifically used search field tags (also termed qualifiers in the Pubmed data base from 1980 until today) using the terms for “cutaneous lupus flares”, “markers”, and/or “immune histopathologic skin lesion due to medication”; the response yielded few reports. Minimal data was available on features in the skin during flares of systemic lupus erythematosus (SLE). Here we describe a patient with chronic, controlled lupus that experienced flares concomitant with medications used for an unhealed ulcer on her leg.
CASE REPORT
We describe a 53 year old Caucasian female who presented to the dermatologist for the sudden presence of an annular, scaly, itchy eruption with erythematous plaques and some raised ridges over the entire body, especially affecting the trunk, arms, and the face. She had a history of 14 years of systemic lupus erythematosus (SLE) treated with tacrolimus 0.5% topically, prednisone 10 milligrams daily orally, Plaquenil® (hydroxychloroquine) tablets of 200 milligrams/day, and iron. In addition, she was taking multiple medications including carbamazepine, trazodone and triamterene-hydrochlorothiazide. Two new medications had been recently added: sulfasalazine and erythromycin for a “non-healing ulcer on her leg”. Upon presentation of the eruption, some studies were performed, including a metabolic panel showing a low chloride level 96 mEq/L, (97-110 mEq/L), a lymphopenia 9.1 um3, (17-480 um3), a monocytopenia 2.6%, (4-10%) and a granulocytosis 88.3103/mm3 units, (1.2-6.8 103/mm3 units). The diagnosis of a drug induced lupus flare in a patient with classic SLE was made, and the following medications were added: Verdeso® (desonide) foam, Vanos®, (fluocinonide), Claritin® (loratadine) and Atarax® (hydroxyzine).
Skin Biopsies
Skin biopsies were taken for hematoxylin and eosin staining (H&E), direct immunofluorescence (DIF) and for immunohistochemistry (IHC) as previously described [8–11]. For the DIF we classified our findings as previously described [3–6], i.e., negative (-), weakly positive (+), positive (+++) and strongly positive (++++).
IHC
We performed IHC as previously described [8–11], utilizing multiple monoclonal and polyclonal antibodies including rabbit anti-human IgG (code AR0423), fibrinogen (code F0111), IgD (code IR517; Southern Biotechnology, Birmingham, Alabama, USA), IgE (code A0094; Vector Labs, Bellingham, Washington, USA), and myeloperoxidase, all from Dako (Carpinteria, California, USA) unless noted. We utilized the following Dako mouse anti-human monoclonal antibodies: CD8, clone C8/144B, CD31, clone JC70A, CD68, clone EBM11, and myeloperoxidase (MPO).
Statement of Ethics
Institutional Review Board (IRB) approval for a case report is not needed. However, the US Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy Rule restricts how protected health information (individually identifiable health information) on a patient is protected. Compliance with patient privacy, institutional rules, and federal regulations were followed. No photos or illustrations that contain identifiable features are included the case report, and the case(s) described in the report are not so unique or unusual that it might be possible for others to identify the patients involved.
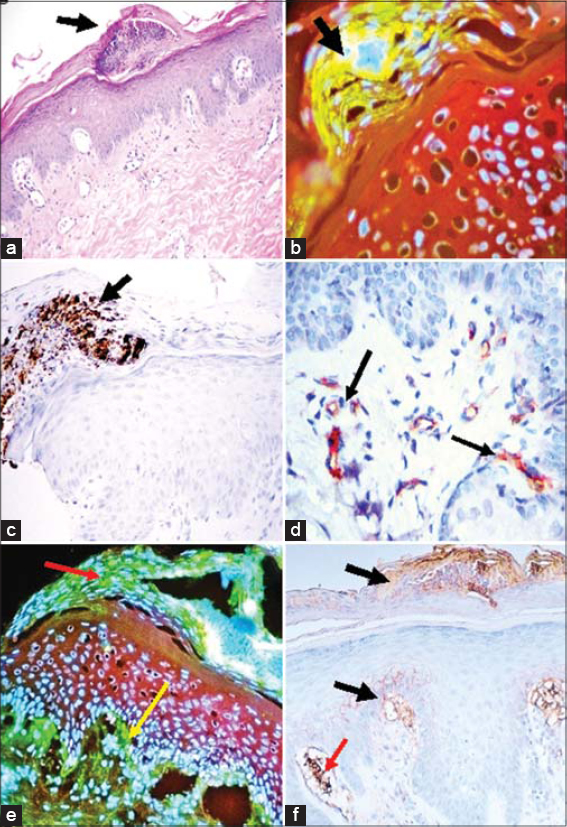
The H&E staining demonstrated mild epidermal hyperkeratosis with minimal follicular plugging and focal subcorneal blisters containing neutrophils and some eosinophils. No evidence of bacteria or fungi was observed (Fig. 1a). Occasional necrotic keratinocytes were also noted in the epidermis. A mild interface infiltrate of lymphocytes and histiocytes was seen in the dermis. Periadnexal infiltrates were also visualized around eccrine glands, as was a perivascular infiltrate. Small blisters were also seen in the basement membrane zone (BMZ).
On DIF examination, the corneal layer (especially in the upper side of the blister), was positive with C3C, fibrinogen IgD, IgA and IgM (+++). Deposits of IgG, IgA, IgM, and IgE were seen at the basement membrane zone (BMZ) (+) in the typical lupus band stain for SLE. IgE was seen around the upper dermal vessels and well as around cell junctions in the epidermis (+++). Fibrinogen and C3C were also positive (in a shaggy pattern at the BMZ) (+++). Cytoid bodies positive for IgG, IgM, IgE and IgA (++) were observed (Fig. 1b, black arrow; 200X and Fig. 1e, red arrows; 100X). The upper dermal vessels were positive with fibrinogen (++++), and IgD. The eccrine glands ducts were positive with fibrinogen (++++), and IgE (++).
With IHC, MPO was strongly expressed on sides of the nascent corneal blisters (+++) (Fig. 1c). Fibrinogen was positive in the corneal blister (+++), and also in both upper and lower neurovascular plexus in the dermis (+++). Using double color IHC, positive staining for IgD was observed in the corneal layer as well as in the vessels, colocalizing with CD31 in the dermal vessels (Fig. 1d). CD8 was positive in the inflammatory infiltrate around dermal vessels at several levels but more strongly around the vessels near the eccrine ducts. Fibrinogen was positive in the dermal papillae, at the adjacent BMZ as well as in the upper dermis. The vessels showed polarization towards the dermal papillae, shown using CD31 in the vessels. CD68 was mostly negative, as well as CD4. Summarizing the main findings, we noted IgA, MPO and fibrinogen deposits in the corneal blisters and IgD, IgE and fibrinogen in the dermal vessels.
DISCUSSION
Lupus flares often present when exacerbating factors occur in patients with known autoimmune lupus [1,8–12]. In our case, this patient was taking multiple medications and the new ones (added for her ulcer; sulfasalazine and erythromycin) are well known of being associated with lupus flares. Fortunately, neither serositis, lung and/or joint symptoms that can be often seen in lupus flares were present [1,9].
The most common known triggers for a lupus flare include ultraviolet light, sulfa drugs, which make a person more sensitive to the sun, such as: trimethoprim-sulfamethoxazole; diuretics and sun-sensitizing tetracycline drugs such as minocycline, penicillin or other antibiotic drugs such as amoxicillin and ampicillin. Other possible triggers and/or flare factors include emotional or physical trauma, weakness, viral infections, cold weather, winter low vitamin D, cigarette smoke, alcohol, occupational exposures (silica, mercury, pesticides, and solvents), hormones, pregnancy and vaccinations. Drug-induced lupus erythematosus flares usually resolve within days to months after removal of the trigger drug (s) in a patient with lupus [1,8–12]. Leg ulcers occur in systemic lupus erythematosus (SLE) due to vasculitis, antiphospholipid antibodies, and, rarely, pyoderma gangrenosum or calcinosis cutis [2,4].
In our case, the medications given for the patient’s ulcer were likely the triggering factor. Of interest the presence of IgE around the vessels and in the cell junctions of the dermis are indicative of some form of allergic reaction to the new medications given to the patients. Recent studies using drug-specific immunoglobulin E (IgE) antibodies have been reported to be have a potential correlation with the putative triggering factors. The authors propose that this test could be modified and applied to assess drug-specific IgE antibodies in the event of drug-related anomalies such the one presented in our case [13].
Based on our findings, skin lesional drug induced lupus flares show differences with lupus. In lupus is common to see deposits of fibrinogen mostly at the BMZ, part of the “lupus band”. Here, and in other cases [9], we also appreciated deposits of immunoglobulins, complement and MPO in the corneal layer above nascent blisters, as well as in the dermal vessels. We speculate that the epigenetic response is created by an inflammatory shift, specifically induced by the flaring agents.
We conclude that in lupus patients with drug induced flares caused by new medications, the presence of IgE in lesional skin should be added as a tool to augment the classic lupus band. As in our case, other biomarkers such other immunoglobulins, complement, fibrinogen and MPO may help to confirm the identity of the flare triggers.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
REFERENCES
1. Clementz GL, Dolin BJ. Sulfasalazine-induced lupus erythematosus. Am J Med. 1988;84:535-38.
2. Abreu Velez AM, Howard MS. Lupus:a comprehensive review. In:Lupus:symptoms, treatment and potential complications. Editors:Thiago Devesa Marquez and Davi Urgeiro Neto. 2012. Series:Immunology and Immune System Disorders. Binding:ebook, pub. sate:2012 3rd Quarter, pages:13-53 (NBC-R), ISBN:978-1-62081-098-9, status:AN. Nova Science Publishers, Inc. 400 Oser Avenue, Suite 1600, Hauppauge, NY 11788, 2011. ISBN:978-1-63482-226-8.
3. Abreu-Velez AM, Klein AD, Howard MS. Specific cutaneous histologic and Immunologic features in a case of early lupus erythematosus scarring alopecia.Our Dermatol Online. 2013;4:199-01.
4. Abreu-Velez AM, Howard MS, Brzezinski P. Immunofluorescence in multiple tissues utilizing serum from a patient affected by systemic lupus erythematosus. Our Dermatol Online. 2012;3:36-42.
5. Abreu Velez AM, Upegui-Zapata YA, Valencia-Yepes CA, Upegui-Quiceno E, Mesa-Herrera NR, Jiménez-Echavarria AM, et al. Membrane attack complex (C5B-9 Complex or Mac), is strongly present in lesional skin from patients with endemic pemphigus foliaceus in El Bagre, Colombia. J Cutan Pathol. 2019;46:925-29.
6. Abreu Velez AM, Upegui-Zapata YA, Valencia-Yepes CA, Upegui-Quiceño E, Jiménez-Echavarria AM, Niño-Pulido CD, et al. Nail alterations in patients affected by endemic pemphigus foliaceus in el Bagre, Colombia. Our Dermatol Online. 2019;10:325-28.
7. Monroe EW. Lupus band test. Arch Dermatol. 1977;113:830-40.
8. Mak A, Tay SH. Environmental factors, toxicants and systemic lupus erythematosus. Int J Mol Sci. 2014;15:16043-56.
9. Abreu-Velez AM, Brown VM, Howard MS. A transient drug induced lupus erythematosus – like allergic drug reaction with multiple antibodies. Our Dermatol Online. 2013;4:511-13.
10. Abreu Velez AM, Klein AD, Howard MS. Jam-A, plasminogen and fibrinogen Reactivity in a case of a lupus erythematosus-Like allergic drug reaction to Lisinopril. J Clin Exp Dermatol Res. 2012;S:6.
11. Crow MK, Olferiev M, Kirou KA. Identification of candidate predictors of lupus flare. Trans Am Clin Climatol Assoc. 2015;126:184-96.
12. Kelati A, Gallouj S, Meziane M, Mernissi FZ. Lupus and pulmonary nodules consistent with bronchiolitis obliterans organizing pneumonia induced by carbamazepine in a man. Our Dermatol Online. 2016;7:445-7.
13. Zhong ZD, Jiang LL, Khandelwal P, Clarke AW, Bakhtiar R, Zou L. Development and utility of an ELISA method for sensitive and specific detection of IgE antidrug antibodies.AAPS J. 2020;22:36
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0002-7301-8274 http://orcid.org/0000-0002-7301-8274 http://orcid.org/0000-0003-0430-6093 http://orcid.org/0000-0003-0430-6093 |



Comments are closed.