Desmoid-type fibromatosis arising in the inguinal region in a young woman: a case report from a histopathological perspective
Martin´s Biopsy Center, Ltd., Martin, Slovakia
Corresponding author: Dr. Vladimír Bartoš
Submission: 16.09.2019; Acceptance: 23.11.2019
DOI: 10.7241/ourd.20203.7
Cite this article: Bartoš V. Desmoid-type fi bromatosis arising in the inguinal region in a young woman: a case report from a histopathological perspective. Our Dermatol Online. 2020;11(3):255-259.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Fibromatoses generally comprise a broad spectrum of myofibroblastic proliferation of similar histomorphology. Desmoid-type fibromatoses (desmoid tumors) are locally aggressive lesions which principally involve deep soft tissue structures. The author describes a 35-year-old female, who was found to have a nodular subcutaneous resistance arising in the upper portion of the right inguinal region. The tumor measured 40 x 25 x 15 mm and adhered to the aponeurosis of the abdominal internal oblique muscle. Histology revealed a dense mass of proliferating spindle-shaped myofibroblasts of uniform appearance. At the periphery, the lesion was poorly circumscribed and infiltrated an adjacent striated muscle. A diagnosis of desmoid-type fibromatosis was made. Desmoid tumor is a quite rare oncologic entity. Although it never metastasizes, it can lead to significant morbidity due to locally aggressive behaviour with a striking tendency to recur. Every patient once treated for deep fibromatosis requires long-term follow-up.
Key words: Desmoid-type fibromatosis, Desmoid tumor, Beta-catenin
INTRODUCTION
Fibromatoses generally comprise a broad group of myofibroblastic proliferation of similar histomorphology whose biologic behaviour is intermediate between that of benign fibrous lesions and fibrosarcomas [1]. According to the WHO (World Health Organisation) classification of tumors [2], they are categorized as neoplasms of uncertain behavior (codes 8813/1, 8821/1 and 8822/1). Various pathologic entities that represent this group occur mostly in adults with a predilection for certain body sites. Based on anatomical distribution, fibromatoses are divided into two major groups with several subdivisions (Table 1) [1]. Superficial (fascial) fibromatoses are slowly growing and of small size. They usually arise from the fascia or aponeurosis. In contrast, deep (musculoaponeurotic) fibromatoses are rapidly growing tumors that often attain a large size, but do not metastasize [1]. As the name indicates, they arise from musculoaponeurotic stromal elements and mainly involve deep soft tissue structures. The synonyms for deep fibromatoses are desmoid tumors (desmoids) or aggressive fibromatosis. The former denomination is derived from a macroscopic appearance of lesions, as the term “desmoid” originates from the Greek word “desmos”, meaning tendon-like. The term aggressive fibromatosis emphasizes a biological behavior that tends to be more aggressive, accompanying by a high recurrence rate [1–3]. Desmoid tumors are quite infrequent in routine clinical practice. In this journal, two articles addressing a superficial [4] and an intraabdominal fibromatosis [5] have been published. In the present paper, a case of subcutaneous desmoid-type fibromatosis in a young woman is described.
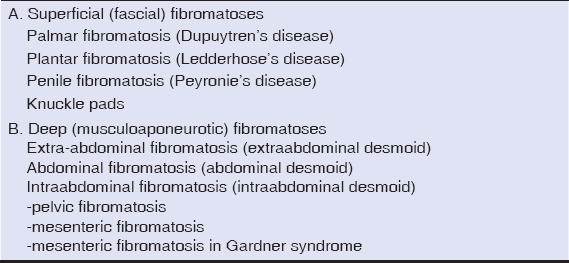 |
Table 1: The anatomical location-based classification of fibromatosis. (from ref. 1) |
CASE REPORT
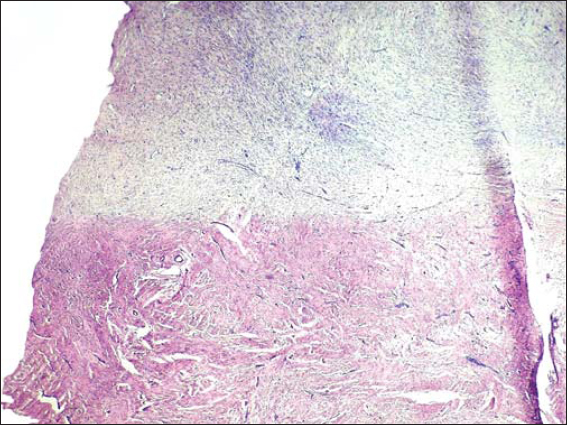
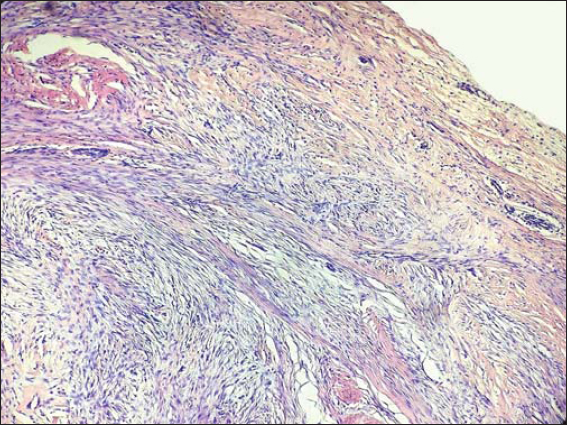
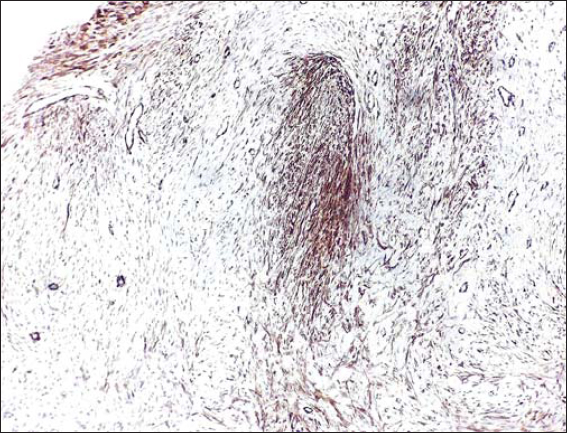
A 35-year-old female manifested with a subcutaneous resistance arising in the upper portion of the right inguinal region. She claimed that the lesion had been present for a few months, during which it had increased in size. On physical and CT examination, it appeared as a nodular subcutaneous tumor mass measuring 3-4 cm in diameter. It stuck to aponeurosis of the abdominal internal oblique muscle. An overlying skin was intact. The presumptive clinical diagnosis was a benign tumor of the soft tissue. A surgical extirpation of the lesion was done following a plastic reconstruction of the inguinal canal. Grossly, the solid tumor measured 40 x 25 x 15 mm, it was white-grayish in color and elastic in consistency. Histology revealed a dense mass of proliferating spindle-shaped cells that was tightly fixed with the aponeurosis (Fig. 1). The proliferation consisted of elongated (myo)fibroblasts of uniform appearance without hyperchromasia or atypia (Figs. 2 and 3). The nuclei were small, sharply defined, some with inconspicuous nucleoli. Mitotic rate was very low (1 mf per 10 HPF) and proliferation activity (Ki-67 index) did not exceed 5%. The tumor cells were usually separated from one another by sparse collagen fibers, but many sections with an extensive hyalinization or keloid-like structures were also visible. Although the cellularity varied from area to area, overall, the lesion was rich in cells. An interesting feature were remnants of degenerated striated muscle cells forming multinucleated giant cell aggregates, that were entrapped within the tumor tissue (Fig. 4). At the periphery, the lesion was poorly circumscribed and infiltrated an adjacent striated muscule. By immunohistochemistry, the tumor cells showed a focal cytoplasmic positivity for alpha-smooth muscle actin (Fig. 5) and almost diffuse nuclear positivity for beta-catenin with moderate to strong staining intensity (Fig. 6). Based on the histopathological findings and immunophenotype, a diagnosis of desmoid-type fibromatosis was made. Because the patient came from another district and was managed in her permanent residency region, no information about her further clinical workup was available at the time of writing this article.
DISCUSSION
Desmoid tumors (DTs) account for 0.03% of all neoplasms [3,6] and less than 3% of all soft tissue tumors [3]. The estimated annual incidence in the general population is 2 – 4 cases per million inhabitants [3,6]. Most DTs arise sporadically, whereas a minor proportion (ca. 8%) is associated with familiar adenomatosis polyposis/Gardner syndrome [3,7]. Mutations in either the APC (adenomatous polyposis coli) or beta-catenin genes are likely to be a major driving force in the formation of DTs and progression of disease [6]. DTs may be found at virtually any body site. In a study conducted by Zreik et al. [7], among 165 individuals with deep fibromatosis, extraabdominal tumors were the most frequent (68%) followed by abdominal wall (16%). The anatomic distribution and gender predominance of the lesions are age-related. In children, DTs occur with equal frequency in men and women, and most are extra-abdominal. Individuals between puberty and the age of 40 tend to be female, and the abdominal wall is the favoured site of involvement. Later in adulthood, these tumors are equally distributed between extra-abdominal and abdominal locations and develop equally in both genders [2]. The clinical course of DTs is quite variable [6]. Although unable to metastasize, these highly infiltrative and locally destructive lesions have a significant tendency to recur, with recurrence rates ranging from 20 to 39% [6].
As it has been demonstrated in the present case, desmoid-type fibromatosis usually consists of the fascicles with slender, spindled cells of uniform appearance set in a collagenous stroma [1,2]. However, the histopathologic pictures may vary between individual lesions. Zreik et al. have recognized seven distinct morphologic patterns: conventional, hypocellular/hyalinized, staghorn vessel, myxoid, keloidal, nodular fasciitis-like, and hypercellular. In their study [7], the mean number of patterns per case was two, with some lesions exhibiting up to five patterns. Of note, abdominal and extraabdominal tumors had a significantly higher percentage of the conventional pattern compared with intraabdominal tumors. Conversely, intraabdominal lesions had a significantly higher percentage of the keloidal. Awareness of the spectrum of histologic patterns is essential to prevent misdiagnosis, especially in small biopsy samples with limited tissue volume. In challenging cases, immunohistochemistry and molecular analysis may help to clarify the diagnosis [7]. In desmoid tumors, the cells stain variably for muscle specific actin and smooth muscle actin, and are typically negative for desmin, h-caldesmon and S100 protein [2]. Of importance, virtually all deep fibromatoses have somatic beta -catenin or adenomatous polyposis coli (APC) gene mutations leading to intranuclear accumulation of beta-catenin [8]. As a result, the vast majority (80-100%) of them show nuclear positivity for beta-catenin detected by immunohistochemistry [8–10]. In 2005, Bhattacharya et al. [8] investigated 21 deep fibromatosis cases with monoclonal beta-catenin antibody and compared with a plethora of other mesenchymal lesions of similar histomorphology, such as low-grade fibromyxoid sarcoma, leiomyosarcoma, various other fibrosarcoma variants, myofibroma/myofibromatosis, nodular fasciitis, and scars. While all examples of deep fibromatosis displayed nuclear beta-catenin staining, all other lesions lacked it, showing only cytoplasmic accumulation. They concluded [8] that beta-catenin immunohistochemistry could separate deep fibromatosis from entities in the differential diagnosis. However, this somewhat contradicts with the study of Carlson and Fletcher [9], who detected nuclear beta-catenin immunopositivity in 80% of cases of sporadic deep fibromatosis, but also in a lower percentage of superficial fibromatoses, low-grade myofibroblastic sarcomas, solitary fibrous tumors, infantile fibrosarcomas, desmoplastic fibrosarcomas, and gastrointestinal stromal tumors. They stated [9], that nuclear staining for beta-catenin was supportive, but not definitive for the diagnosis of desmoid fibromatosis.
Since beta-catenin signalling pathways plays a key role in the pathogenesis of this disease, there have been attempts to disclose a potential prognostic importance of beta-catenin immunoreactivity in the tumors. However, several studies have demonstrated conflicting results until now [10–12]. In a recent paper of Korean authors [11], the 5-year progression free survival rate was 100% in the group with a high nuclear beta-catenin intensity and 62.5% in the group with a low nuclear beta-catenin intensity, although showing no significant difference. Hamada et al. [12] demonstrated that a higher nuclear expression of beta-catenin was significantly associated with a poor response to meloxicam treatment. Other investigators have not found a significant correlation between nuclear beta-catenin expression and the outcome of COX-2 inhibitor therapy [10]. To answer the question whether immunohistochemical evaluation of this marker could have a prognostic value in clinical practice might require further studies.
CONCLUSION
Desmoid-type fibromatosis is a quite rare oncologic entity. Although this tumor never metastasizes, it can lead to significant morbidity due to locally aggressive behaviour with a striking tendency to recur. Every patient once treated for deep fibromatosis requires long-term follow-up. Nuclear beta-catenin expression in tumor cells is an important immunohistochemical feature for differential diagnosis, but further research is needed to elucidate whether it may also serve as a predictive marker.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
REFERENCES
1. Weiss SW, Goldblum JR (Eds). Enzinger and Weisss Soft Tissue Tumors. 4th edition. Mosby, Inc. 2001;1622 p. ISBN 0-323-01200-0.
2. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (Eds). WHO Classification of Tumours of Soft Tissue and Bone. IARC:Lyon 2013;468 p. ISBN 978-92-832-2434-1.
3. Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol. 2012;23:562-9.
4. Sadr Eshkevari Sh, Nickhah N. Ledderhose disease:A case report with palmar fi bromatosis, keloid and partial response to oral retinod. Our Dermatol Online. 2015;6:52-5.
5. Vidyasagar R, Sudarshan P, Suresh S, Bhat V, Subramanya M. Rare case report of mesenteric fibromatosis. Our Dermatol Online. 2015;6:443-6.
6. Ganeshan D, Amini B, Nikolaidis P, Assing M, Vikram R. Current update on desmoid fibromatosis. J Comput Assist Tomogr. 2019;43:29-38.
7. Zreik RT, Fritchie KJ. Morphologic spectrum of desmoid-type fibromatosis. Am J Clin Pathol. 2016;145:332-40.
8. Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C, Ricci F, Weber K, Furlong MA, et al. Nuclear beta-catenin expression distinguishes deep fibromatosis from other benign and malignant fibroblastic and myofibroblastic lesions. Am J Surg Pathol. 2005;29:653-9.
9. Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cell lesions:analysis of a series and review of the literature. Histopathology. 2007;51:509-14.
10. Sakai T, Nishida Y, Hamada S, Koike H, Ikuta K, Ota T, et al. Immunohistochemical staining with non-phospho β-catenin as a diagnostic and prognostic tool of COX-2 inhibitor therapy for patients with extra-peritoneal desmoid-type fibromatosis. Diagn Pathol. 2017;12:66.
11. Kim JS, Kim HJ, Lee MY, Moon KC, Song SG, Kim HS, et al. Survival outcomes after adjuvant radiotherapy for aggressive fibromatosis depend on time frame and nuclear β-catenin. Radiat Oncol J. 2019;37:37-42.
12. Hamada S, Urakawa H, Kozawa E, Futamura N, Ikuta K, Shimoyama Y, et al. Nuclear expression of β-catenin predicts the efficacy of meloxicam treatment for patients with sporadic desmoid tumors. Tumour Biol. 2014;35:4561-6.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|






Comments are closed.