A case of erysipelas during secukinumab therapy in a patient with HTLV-1-positive psoriatic arthritis
Yasunobu Kato, Toshiyuki Yamamoto
Department of Dermatology, Fukushima Medical University, Fukushima, Japan
Corresponding author: Prof. Toshiyuki Yamamoto, E-mail:
Submission: 08.02.2020; Acceptance: 16.04.2020
DOI: 10.7241/ourd.20203.27
Cite this article: Kato Y, Yamamoto T. A case of erysipelas during secukinumab therapy in a patient with HTLV-1-positive psoriatic arthritis. Our Dermatol Online. 2020;11(3):325-326.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
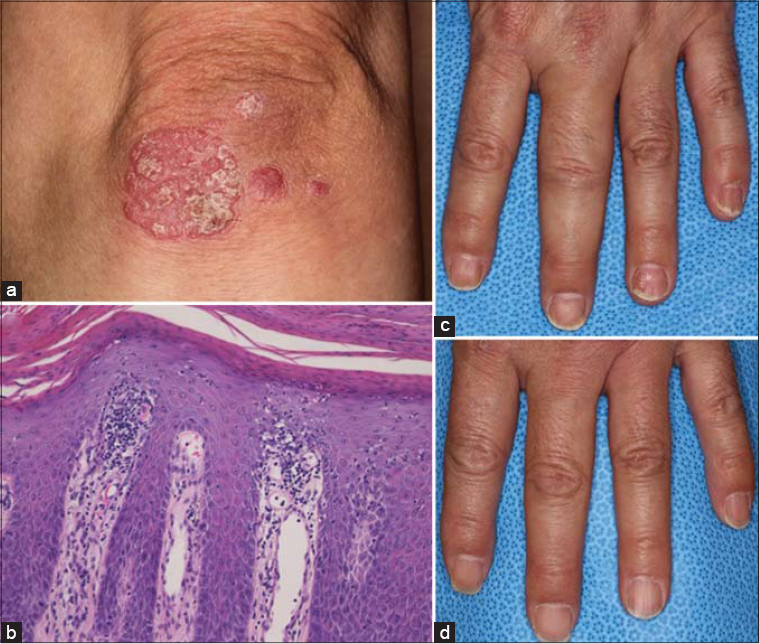
A 55-year-old male had suffered from joint pain for several years, with psoriatic plaques developed one and a half years before. Physical examination revealed keratotic erythematous plaques on the scalp, trunk, and extremities (Fig. 1a). The Psoriasis Area and Severity Index (PASI) score was 8.6. Furthermore, dactylitis was observed in the left third finger (Fig. 1b). A biopsy specimen taken from the scaly erythema on the elbow revealed hyperkeratosis with parakeratosis, a regular elongation of the epidermis, and mononuclear cell infiltration around the dilated blood vessels in the papillary layer (Fig. 1c). Laboratory examination showed a human T-cell lymphoma virus type 1 (HTLV-1) infection (99.82 S/CO by CLIA), which was confirmed by a Western blot. An examination of the HTLV-1 provirus in the skin was negative. Therapy with subcutaneous secukinumab injections into the abdomen was introduced (300 mg at weeks 0, 1, 2, 3, 4; and every 4 weeks thereafter) under careful supervision. Four months after the initial administration, a clear PASI score was achieved, and joint pain was in remission with an improvement in dactylitis (Fig. 1d). After the ninth injection, the patient wished to reduce the dose of secukinumab to 150 mg because of economic concerns. One month after the first injection of 150 mg secukinumab, the patient complained of painful redness in the left ear. Physical examination revealed that the erythema had spread and coalesced diffusely in the left ear (Fig. 2). Laboratory examination showed an elevated concentration of white blood cells (9,100/µL with 70% neutrophil, 18% lymphocyte, 11% monocyte, and 1% basophil), C-reactive protein (4.88 mg/dL), and antistreptolysin O (195 IU/mL), whereas renal and liver functions were normal. Secukinumab was stopped, and oral amoxicillin trihydrate-potassium clavulanate was administered instead (250 mg/day for 5 days). After an improvement in erysipelas, secukinumab was restarted. Thereafter, the patient has been well-controlled without a recurrence of erysipelas.
Biologics should be administered carefully in immunosuppressive patients with hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection [1]. Our patient was HTLV-1–positive, but the HTLV-1 provirus was not detected in the psoriatic skin by a Southern blot analysis. To date, there have only been a few reports of the use of biologics in HTLV-1 carrier patients with psoriasis [2,3]. In the case studied, four months after starting secukinumab, a clear PASI score was achieved. In addition, dactylitis of the fingers significantly improved and joint pain subsided. Because of economic concerns, however, the dose of secukinumab was reduced. Although skin and joint manifestations remained well-controlled, the patient developed erysipelas in the left ear. To date, there have been several cases of erysipelas during biologic treatment, but only a handful of cases of erysipelas during secukinumab therapy have been reported [4].
IL-17 recruits leukocytes, such as neutrophils, to infection sites, and aids defense mechanisms in the fight against extracellular bacteria. Neutropenia was reported infrequently in patients receiving secukinumab, and most cases were grade 1 or 2, and transient [5]. Among patients treated with 300 mg and 150 mg secukinumab, there was no significant variation in the occurrence of neutropenia of grade 2 or greater [6]. Serial infections were infrequent. Although this patient developed a mild infection, HTLV-1 positivity may be relevant to the induction of erysipelas. Caution may, thus, be necessary in the administration of biologics in patients with HTLV-1.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients have given consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Kaushik SB, Lebwohl MG. Which therapy for which patient:Focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
2. Kazama M, Umezawa Y, Itoh M, Nobeyama Y, Ito T, Kikuchi S, et al. Successful treatment of ustekinumab in a psoriasis patient with human T-cell leukemia/lymphotropic virus type 1 infection. J Dermatol. 2017;44:1334-5.
3. Hamada K, Sawada Y, Hino R, Nakamura M. HTLV-1 carrier psoriasis patients treated by anti-IL-23/IL-17. Australas J Dermatol. 2018;59:e154.
4. Zarza LP, Morrondo CD. Facial erysipelas after secukinumab therapy. Reumatol Clin. 2018.pii:S1699-258X(18)30171-2.
5. Blauvelt A. Safety of secukinumab in the treatment of psoriasis. Exp Opin Drug Safety. 2016;15:1413-20.
6. Van der Kerkhof PCM, Griffiths CEM, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience:a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-8390-2573 http://orcid.org/0000-0002-8390-2573 |




Comments are closed.