Seborrheic keratoses and concomitant malignant tumors: the controversy
Francisco Gil , Joana Parente, João Aranha
, Joana Parente, João Aranha
Department of Dermatology, Hospital de Santarém EPE, Santarém, Portugal
Corresponding author: Dr. Francisco Gil, E-mail:
Submission: 16.10.2019; Acceptance: 27.12.2019
DOI: 10.7241/ourd.20203.23
Cite this article: Gil F, Parente J, Aranha J. Seborrheic keratoses and concomitant malignant tumors: the controversy. Our Dermatol Online. 2020;11(3):316-318.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Seborrheic keratosis (SK) is the most common benign epidermal tumor [1,2]. Considering its prevalence, reports of concurrent malignant tumors within SKs are rare, but have added to the debate on the possibility of the development of these neoplasms from SK [3–5]. We briefly describe two clinical cases illustrative of the association of SK with concomitant malignant neoplasms and analyze the controversy over whether they are of incidental coexistence or represent malignant transformation.
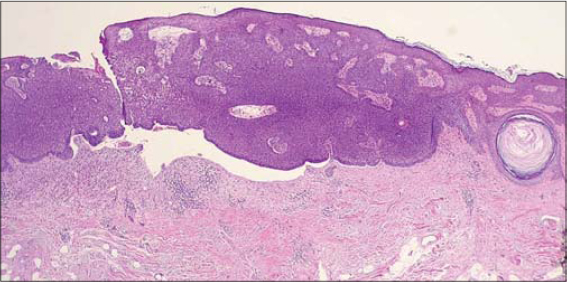
The first case concerns a 75-year-old Caucasian female with no relevant dermatological background who presented an asymptomatic violaceus erythematous papule arising adjacent to a larger (35×20 mm), well-demarcated, elevated, flat, brownish plaque on the right frontal area (Fig. 1). The patient noticed the brownish plaque many years prior to the examination while the violaceus erythematous papule developed much later. Dermoscopy revealed a brown lesion with comedo-like openings and, peripherally, a shiny, brownish-red area with arborizing vessels, superficial fine telangiectasia, ulceration, and bluish-gray globules (Fig. 2). The newly developed papule was excised. A histopathological analysis of the papule and the brownish plaque revealed a basal cell carcinoma with large nests of basaloid cells and superficial components in collision with an acanthotic SK, respectively (Fig. 3).
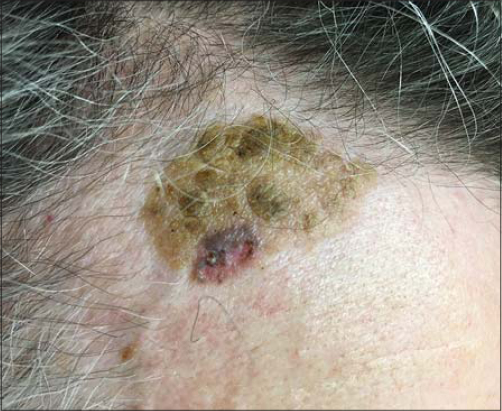
The second case concerns a 66-year-old female who presented with recent changes in a long-lasting, slightly elevated, 80×40 mm, brownish plaque on the left flank. A previous incisional biopsy of the lesion revealed the presence of an SK. However, over the past year, the central area 15 mm in diameter had become persistently erythematous, pruritic, and hemorrhagic after rubbing (Fig. 4).
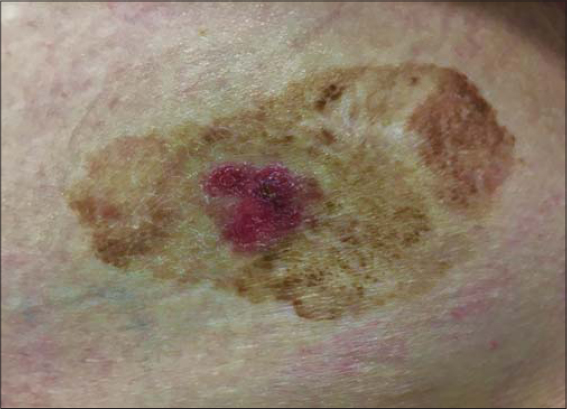 |
Figure 4: Clinical view of the lesion: a recently developed central erythematous area in the previously biopsied SK. |
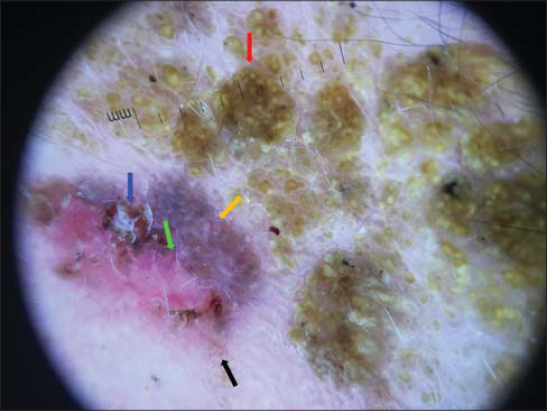
An incisional biopsy of this area was performed revealing a well-differentiated in situ squamous cell carcinoma (Fig. 5). A wider excision was performed, removing the recently developed part of the lesion completely and showing the same histopathologic findings contiguous with an SK.
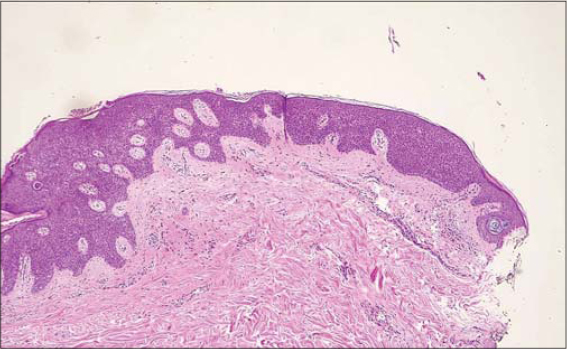 |
Figure 5: Histologic examination: a well-differentiated in situ squamous cell carcinoma (hematoxylin and eosin, x40). |
A malignant tumor within an SK is an extremely rare condition [2,6] and the histological subtype involved is usually acanthotic SK [7]. Although the most commonly developed type of malignant tumor is basal cell carcinoma—mainly its superficial type [6,7]—squamous cell carcinoma, eccrine porocarcinoma, and malignant melanoma have also been reported [3]. The relationship between these tumors and SK is still a matter of controversy, and the debate as to whether this exceedingly rare association is incidental or generated by tumor progression continues [2]. Some authors maintain that it is a matter of chance, doubting the existence of any pathogenic relationship between malignant tumors and SK and defending the incidental cohabitation of the two entities [5]. The term collision tumor is applied to the coexistence of two or more different neoplasms, benign or malignant, in the same cutaneous lesion, while the term compound tumor refers to two distinctive neoplasms either directly contiguous or immediately adjacent to each other [3].
Other authors defend the hypothesis of malignant transformation, arguing that SKs are composed of several cell types and the transformation to a variety of epithelial tumors derives from these individual cells. Therefore, basal cell carcinomas could arise from basaloid cells, squamous cell carcinomas from squamous cells, and melanomas from melanocytes, all within an SK [1]. The histological evidence of a direct connection between the two coexisting lesions might be indicative of a malignant transformation of the SK. It has been proposed that, when malignant lesions are not merely adjacent to or merging with an SK, but are found within, the association may be more than coincidental, supporting the view for potential dysplastic or malignant change [7].
We report two cases illustrative of the unsettled controversy of the coexistence of SKs and malignant tumors: incidental coexistence vs. subtle malignant transformation. In the first case studied, the apparent coexistence of two adjacent, but histologically distinct, tumor components opposes the development of a malignant lesion within the SK examined in the second case, which, according to some authors, is indicative of the possibility of malignant transformation in so-called benign lesions. We also intend to underscore the importance of a low threshold for biopsies of suspicious SK lesions if there exists a modification of macroscopic characteristics and in the presence of atypical clinical symptoms such as erythema, ulceration, bleeding, and crusting, as they may herald a malignant tumor.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients have given consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Smolyannikova VA, Alexandrova AK. Multiple seborrheic keratosis in a patient with familial benign pemphigus. Our Dermatol Online. 2017;8:289-92.
2. Fusco N, Lopez G, Gianelli U. Basal cell carcinoma and seborrheic keratosis:when opposites attract. Int J Surg Pathol. 2015;23:464.
3. Bedir R, Yurdakul C, Guçer H, Sehitoglu I. Basal cell carcinoma arising within seborrheic keratosis. J Clin Diag Res. 2014;8:YD06-7.
4. Sousa C, Podlipnik S, Puig S, Malvehy J. When seborrheic keratosis Is wearing a mask. Dermatol Pract Concept. 2020;10:e2020038.
5. Jee H, Lee N, Ahn S. Case of seborrheic keratosis with underlying basal cell carcinoma suggesting a collision tumor. J Dermatol. 2013;40:837-9.
6. Cimpean I, Theate I, Vanhooteghem O. Seborrheic keratosis evolution into squamous cell carcinoma:A truly modified sun-related tumor?A case report and review of the literature. Dermatol Reports 2019;11:7999.
7. Oka M, Yamamoto Y, Fujii M. Transformation of seborrheic keratosis into bowenoid actinic keratosis via three steps of histological change in a patient with rheumatoid arthritis treated with multiple immunosuppressants. Case Rep Dermatol 2020;12:19-24.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.