Atypical paraneoplastic pemphigus associated with pulmonary adenocarcinoma
Almamy Diabaté , Ali Naqi, Julien Pittoni, Romain Prud’homme, Assikar Safae, Iona Matei, Christophe Bedane
, Ali Naqi, Julien Pittoni, Romain Prud’homme, Assikar Safae, Iona Matei, Christophe Bedane
Unit of Dermatology, University Hospital of Dupuytren, and CNRMBAI, Limoges, France
Corresponding author: Dr. Almamy Diabaté, E-mail: docalmamy@yahoo.fr
Submission: 31.03.2019; Acceptance: 02.08.2019
DOI: 10.7241/ourd.20194.7
Cite this article: Diabaté A, Naqi A, Prud’homme R, Safae A, Matei I, Bedane C. Atypical paraneoplastic pemphigus associated with pulmonary adenocarcinoma. Our Dermatol Online. 2019;10(4):349-351.
Citation tools:
BibTex | CSV | RIS | refer/BiblX | Endnote XML
Copyright information
© Our Dermatology Online 2019. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Paraneoplastic pemphigus (PNP) is a muco-cutaneous autoimmune disease associated with several types of internal malignancy. We report a case of a 57-year-old male with an atypical form of PNP associated with pulmonary adenocarcinoma. There was no involvement of the mucous membranes. Although the macroscopic and histological appearances were typical of a pemphigus, the direct immunofluorescence was positive for the IgG staining and the C3 intercellular. Circulating antibodies were detected with envoplakin, periplakin and desmoplakins 1 and 2; but the Ac anti desmoglein 1 IgG and 3 IgG, BP 180 and BP 230 and auto Ac anti-epidermis were negative. The clinical evolvement was observed with a combination of systemic corticosteroids and radiotherapy associated with Cisplatin-Alimta chemotherapy.
Key words: Paraneoplastic pemphigus, Pulmonary adenocarcinoma
INTRODUCTION
Paraneoplastic pemphigus (PNP) is a distinct autoimmune disease that is associated with underlying malignancy including lymphoproliferative diseases and adenocarcinoma. The typical form of PNP occurs most often between 45 and 70 years of age, with floral lesions of the oral mucosa, a generalized polymorphic rash. His diagnosis is based on investigations including tumour identification, histopathological studies, immunofluorescence and serum immunological studies [1–3]. The atypical PNP has been rarely described in the literature [4]. We report a case of atypical PNP with no mucosal lesions associated with pulmonary adenocarcinoma in a 57-year-old male.
CASE REPORT
A 57-year-old patient of phenotype 2, active smoker (40 packs a year) consulted in the dermatology unit at Dupuytren Hospital in Limoges in January 2015 for a pustular palmoplantar rash associated with pruritus which was treated with dermo-corticoid and acitretin (SORIATANE 25) for a probable psoriasis. In his antecedents, he had a tonsillectomy in 2005, and he is allergic to aspirin. In September 2015, the pustular rash became widespread reaching the nails, palms of the hands (Fig. 1), and the sole of the foot, accompanied by impetiginization without mucosal involvement. The para-clinical investigation showed a biologically leukocytosis at 18.3 giga/L and Polynuclear Neutrophils at 11.16 giga/L. The platelet count, the blood ionogram, the hepatic and renal status, LDH, VS and CRP were normal. The electrophoresis of the proteins found a hypoalbuminemia without viewable monoclonal component. The viral serology for HIV, HBV and HCV were negative. The immunological investigation showed a negative indirect immunofluorescence for Ac anti desmoglein 1 IgG and 3 IgG, BP 180 and BP 230, an absence of auto Ac anti-epidermis and antibodies anti-envoplakin, anti-periplakin and desmoplakins 1 and 2 were positive. Histologically, there was a discrete spongiosis, no intra-epidermal vesicles in the epidermis, sub-epithelial detachment, (the bubble cavity filled with many inflammatory elements with Neurophile Polynuclear and Eosinophilic Polynuclear with Vacuolar alteration in the dermal-epidermal junction. In the superficial dermis, there were perivascular lymphocyte infiltrate, polymorphic inflammatory infiltrate (predominantly PNE) (Fig. 2). The direct IgA immunofluorescence was negative, IgG intracellular substance was strongly positive and the factor C3 complement of the intracellular substance was also positive for basal staining. Radiographically, the TAP scanner more cerebral showed a necrotic pathological pulmonary mediastinal and hilar mass. The pet scan confirmed the presence of right para-hilar tumour with ipsilateral mediastinal ganglia, under the coronary and retro-clavicular right wing (Fig. 3). The bronchial endoscopy with mediastinal ganglionic puncture showed the presence of carcinomatous groups, which were suggestive of adenocarcinoma. There was an absence of BRAF, KRAS, HER2 mutations. Considering this bundle of evidence, the diagnosis of a paraneoplastic pemphigus with pulmonary adenocarcinoma was retained. The treatment undertaken was for the pemphigus 0.5 mg/kg/day of prednisone or 40 mg/day and for the pulmonary adenocarcinoma, a radiotherapy and chemotherapy with Cisplatin-Alimta. After a six-month-post-treatment, the skin of the patient returned to normal with a recovery of four kilograms of weight, but there was a persistence of nail lesions of the feet.
 |
Figure 1: Palmar bullous eruption. |
 |
Figure 2: Histology of pemphigus paraneoplasia. |
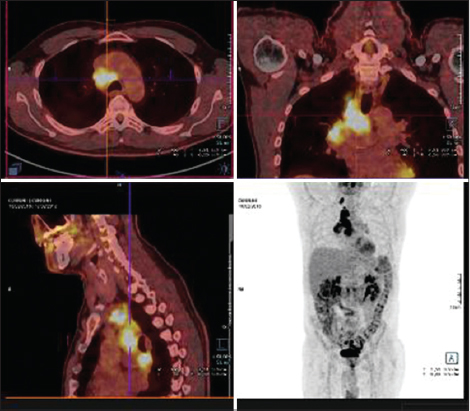 |
Figure 3: Pulmonary adenocarcinoma with PET scan. |
DISCUSSION
The classic PNP described by Anhalt in 1990 in the 45-70 age group predominated in males and the associated diseases were lymphoproliferative diseases and adenocarcinomas. Clinically, erosive and ulcerative mucosal lesions are consistent signs of the disease and skin lesions are polymorphic [1–3]. Our observation is distinguished by the purely cutaneous involvement at the clinical level. This atypical form without mucosal involvement was described by Kennedy [4] in 2009 in a 78-year-old woman with recurrent endometrial cancer. The antibodies desmoglein 1 IgG and 3 IgG, BP 180 and BP 230 were negative; however, the direct immunofluorescence was positive for the inter-cellular IgG and C3 staining and the presence of antibodies anti-envoplakin, anti-periplakin and desmoplakin 1 and 2. This negativity of the indirect immunofluorescence was described by Wong [5]. According to Wong [5], these are modified criteria for the PNP. Moreover, this negation of antibodies anti-desmoglein IgG 1 and IgG 3 is not correlated with isolated mucosal involvement or muco-cutaneous involvement [6]. The tumour associated with the PNP is usually lymphoproliferative tumours [1,7–9]. Some authors [4,5,10] described the PNP with solid tumours as in our observation which shows a pulmonary adenocarcinoma. The evolution of our patient was satisfactory under corticosteroid combined with radiotherapy coupled with Cisplatin-Alimta chemotherapy. Kennedy [4] in his case associated corticosteroids with resection of the tumour. In general, the prognosis is poor, not only because of the possible progression of malignant tumours, but also because the treatment often leads to infectious complications, which is the most common cause of death in PNP patients [3].
CONCLUSION
This observation presents an atypical form of PNP. This case suggests that, in addition to the typical form, other pauci-symptomatic forms may be involved in PNP. Nevertheless, it is the histological and immunological investigations that allow the diagnosis.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Jiang Q, Zhang BH. Paraneoplastic pemphigus associated with chronic lymphocytic leukemia:A case report. Med (Baltimore). 2017;96:6184.
2. Yong AA, Tey HL. Paraneoplastic pemphigus. Australas J Dermatol. 2013;54:241-50.
3. Wieczorek M, Czernik A. Paraneoplastic pemphigus:a short review. Clin Cosmet Investig Dermatol. 2016;23:291-5.
4. Kennedy NA, Dawe S. Atypical paraneoplastic pemphigus secondary to endometrial carcinoma with no mucosal involvement. Clin Exp Dermatol. 2009;34:130-3.
5. Wong KC, Ho KK. Pemphigus with pemphigoid-like presentation, associated with squamous cell carcinoma of the tongue. Australas J Dermatol. 2000;41:178–80.
6. Ohyama M, Amagai M, Hashimoto T, Nousari HC, Anhalt GJ, Nishikawa T. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-8.
7. Taintor AR, Leiferman KM, Hashimoto T, Ishii N, Zone JJ, Hull CM. A novel case of IgA paraneoplastic pemphigus associated with chronic lymphocytic leukemia. J Am Acad Dermatol. 2007;56:S73-6.
8. Nikolskaia OV, Nousari CH, Anhalt GJ. Paraneoplastic pemphigus in association with Castleman’s disease. Br J Dermatol. 2003;149:1143-51.
9. Mimouni D, Anhalt GJ, Lazarova Z, Aho S, Kazerounian S, Kouba DJ, et al. Paraneoplastic pemphigus in children and adolescents. Br J Dermatol. 2002;147:725-32.
10. Zheng S, Gaojie L, Jianping L, Tingfeng F, Yunjie Z, Huayao Z, et al. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma:report of a case and review of literature. Int J Clin Exp Pathol. 2015;8:11983–94.
Notes
Source of Support: Nil
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|


Comments are closed.