|
Get Citation
|
|
|
López-López F, Ponce S, De Velasco G. Toxicoderma by gemcitabine: about a case. Our Dermatol Online. 2018;9(1):25-26. |
|
|
Download citation file:
|
Toxicoderma by gemcitabine: About a case
Flora López-López, Santiago Ponce, Guillermo De Velasco
Department of Medical Oncology, 12 Octubre Hospital, Madrid, Spain
Corresponding author: Dr. Guillermo De Velasco, E-mail: gdvelasco.gdv@gmail.com
Submission: 19.06.2017; Acceptance: 21.08.2017
DOI: 10.7241/ourd.20181.7
ABSTRACT
Skin reactions are often related with drugs, particularly with chemotherapy. Several types of cutaneous involvement have been described from pruritus alone to generalized eruptions not always but generally related with hypersensitivity or inflammatory reactions that usually are independent of primary tumor location. Gemcitabine is a widely used chemotherapy drug that may typically produce a mild skin rash. Herein, we show a case of uncommon and severe direct skin toxicity because of gemcitabine in context of thrombocytopenia. We have also performed a review of the literature showing that this may be the first case of such toxicity.
Key words: Gemcitabine; Rash; Lung cancer
INTRODUCTION
Gemcitabine is a nucleoside analog approved for many tumor types such as bladder, breast, lung or pancreatic. Common toxicities include mild myelosuppression, increase liver enzymes and flu-like symptoms. Skin rash may occur in up to 30% of patients, though grade 3-4 is uncommon [1]. Several types of cutaneous involvement have been described from pruritus alone or generalized morbilliform eruptions [2] to scleroderma-like changes in lower extremities [2,3], generally related with hypersensitivity or inflammatory reactions.
Herein, we show a toxic skin reaction by gemcitabine associated to thrombocytopenic purpura in context of hematological toxicity grade 4 (Common Terminology Criteria for Adverse Events version 4) after first cycle of carboplatin plus gemcitabine. The patient underwent skin biopsy which confirmed diagnostic and oral steroids were given until resolution. This is an unusual case because skin reactions by gemcitabine use to be immune-mediated not for direct cutaneous toxicity and the lesions presented have a particular characteristics and distribution.
CASE REPORT
A 60-year-old white man, former smoker, was given the diagnosis of non-small cell lung cancer with squamous differentiation and liver metastasis. He started first line platinum-gemcitabine regimen. Four days after fist cycle, patient consulted at emergency room with fever and skin lesions on the chest, root of upper limbs and lower limbs slightly itchy, bilateral and symmetric without edema (Figs. 1a and 1b). He did not have other source of infection and he had not taken other drugs.
At physical examination there were no other notable findings but mild bronchospasm.
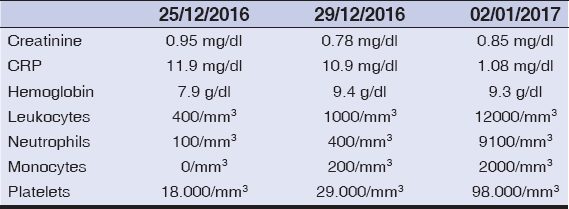
Chest X-ray did not show any infiltrates, urine was normal and blood tests showed grade 4 neutropenia and thrombocytopenia (Table 1). Empirical antibiotic therapy with amoxicillin clavulanic and ciprofloxacin was initiated. Blood transfusions and granulocyte colony-stimulating factor were also required. Dermatology took skin biopsies and patient was on systemic steroids (intravenous prednisone at doses of 0,5 mg/kg/day) with progressive resolution of the lesions. Almost complete hematological recovery was achieved at 8 days, ending the antibiotic cycle without microbiological isolation.
The skin biopsy result and response to steroids confirmed the plausible pharmacological origin of the rash. In the absence of other new drugs, gemcitabine is the most likely responsible agent. After complete resolution of the laboratory tests, the patient was able to continue the treatment with dose adjustment and without resurgence of the rash, although he needed a slow downward steroid regimen for a month after hospitalization.
DISCUSSION
Since its approval, gemcitabine has been reported associated with a limited number of cutaneous reactions (24,8%), rash (20,3%) and urticaria or pruritus [4]. These side effects are usually mild and transient, are resolved spontaneously and rarely resulting in adjustment of the dose. The kind of lesions presented in our patient are not similar to those reported previously [5,6], because of their purpuric features and the circumscribed distribution to the thorax and lower limbs, being generalized in most cases. In this patient the coincidence with thrombopenia and febrile neutropenia is likely to distort part of the symptoms.
We think that the importance of this case is to be aware that despite the long time in use of certain antitumor drugs, there may always be toxicity not previously described that depends on the idiosyncrasy of each patient. In addition to the importance of an adequate differential diagnosis with specific tests (such as biopsy) that may help to guide the diagnosis.
REFERENCES
2. Payne A, Savarese D. Cutaneous side effects of conventional chemotherapy agents. Feb 2017 UpToDate.
3. Bessis D, Guillot B, Legouffe E, Guilhou JJ. Gemcitabine-associated scleroderma-like changes of the lower extremities. J Am Acad Dermatol. 2004;51(2 Suppl):S73-6.
4. Aapro MS, Martin C, Hatty S. Gemcitabine–a safety review. Anticancer Drugs. 1998;9:191-201.
5. Kuku I, Kaya E, Sevinc A, Aydogdu I. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-2.
6. D’epiro S, Salvi M, Mattozzi C, Giancristoforo S, Campoli M, Zanniello R, et al. Gemcitabine-induced extensive skin necrosis. Case Rep Med. 2012;2012:831616.
Notes
Source of Support: Nil
Conflict of Interest: None declared.

Comments are closed.