Rapidly enlarging giant facial mass: Initial presentation of blastic plasmacytoid dendritic cell neoplasm
Gulsen Akoglu1, Sibel Orhun Yavuz2, Aydan Kilicarslan2, Tekin Guney3
1Department of Dermatovenereology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey; 2Department of Pathology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey; 3Hematology Clinics, Ankara Ataturk Training and Research Hospital, Ankara, Turkey
Corresponding author: Dr. Gulsen Akoglu, E-mail: gusemd@yahoo.com
Submission: 09.01.2016; Acceptance: 25.03.2017
DOI: 10.7241/ourd.20174.128
ABSTRACT
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematological malignancy characterized by proliferation of plasmacytoid dendritic cell precursors. Herein, we describe a 65-year-old male presented with a 6 month history of a progressively enlarging purple mass over his forehead. The histopathological examination revealed non-epidermotropic, dermal, and subepidermal homogeneous infiltration with moderate-sized cells resembling lymphoblasts or myeloblasts. The neoplastic clone diffusely stained positive with CD4 and CD56. They were negative for CD3, CD20, pax-5, CD30, myeloperoxidase, and TdT. Rearrangement studies for T and B cell receptor were negative. Ki-67 proliferation index was 80%. Involvement of multiple lymph nodes was detected. Bone marrow biopsy was normal. The patient was put on six cycles of R-CHOP chemotherapy which was successfully cleared all lesions. A rapid recurrence was observed after 1.5 months. While being prepared for autologous stem cell transplantation, the patient died due to rapidly onset myelosuppression and systemic infections. In conclusion, BPDCN should be in the clinical and histopathological differential diagnosis of rapidly developing tumoral cutaneous lesions. Early diagnosis is important to initiate aggressive treatments in BPDCN, in which skin involvement is very frequent.
Key words: Blastic plasmacytoid dendritic cell neoplasm; Differential diagnosis; Skin; Therapy
INTRODUCTION
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematological malignancy. The disease was named as CD4+/CD56+ hematodermic neoplasm or NK cell lymphoma until immunophenotypic and gene expression features of the neoplastic cells showed the resemblance of plasmacytoid dendritic cells (pDC) [1].
BPDCN usually occurs in elderly, especially in men, with a mean age of 60-70 years. The prognosis is poor. Skin involvement is usually present at the diagnosis [2]. Herein, we report an elderly patient having a diagnosis of BPDCN and details of clinicopathological features and the follow up observations.
CASE REPORT
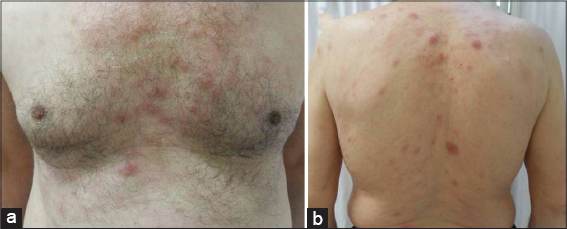
A 65-year-old male presented with a 6 month history of an enlarging purple mass over his forehead. He had undergone a skin biopsy in another health centre before and diagnosed as having atypical T cell infiltration. He was referred us for advanced investigations. The patient was on antihypertensive treatment and he had a 45-pack year history of smoking. In the initial dermatological examination, a 5×4 cm sized and 1 cm elevated firm, purplish mass with overlying telangiectasias and a small crust were observed on the forehead. The otherwise body skin was normal. Organomegaly was not detected. Multiple firm and immobile lymphadenopathies largest measuring 3×3 cm in size were palpated over right cervical area. An incisional skin biopsy was performed from the cutaneous lesion. After obtaining skin biopsy, multiple erythematous nodules and plaques with a diameter ranging from 0.5 to 1.5 cm developed and facial lesion progressively enlarged up to 14 x 12 cm within 2 weeks (Figs. 1 and 2).
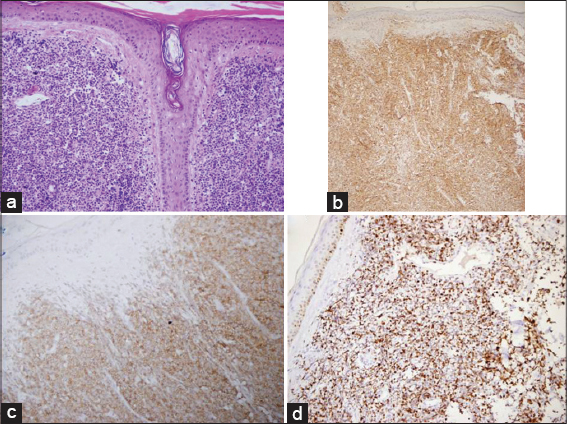
The histopathological examination of the forehead and trunk lesions revealed non-epidermotropic, dermal, and subepidermal homogeneous infiltration with moderate-sized cells resembling lymphoblasts or myeloblasts. The neoplastic clone diffusely stained positive with CD4 and CD56. They were negative for CD3, CD20, pax-5, CD30, myeloperoxidase (MPO), and terminal deoxynucleotidyl transferase (TdT). Receptor rearrangement studies for T and B cell were negative. Ki-67 proliferation index was 80% (Fig. 3). Depending on these features, the patient was diagnosed as having BPDCN.
The patient was consulted to hematology clinic. Total blood count with differential, erythrocyte sedimentation rate, liver and kidney function tests, beta 2 microglobulin and lactate dehydrogenase levels were within normal limits. Bone marrow biopsy was normal. Cranial tomography did not show bone or parenchymal involvement. Thoracoabdominal tomography was normal. Whole body 18F-Fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) imaging revealed increased uptake in soft tissue lesion on forehead, bilateral submandibular, bilateral cervical and right supraclavicular, and bilateral axillary lymph nodes, and increased metabolic activity in parotid glands. The patient was put on six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, and prednisone plus vincristine) chemotherapy which was successfully cleared all lesions. However, a rapid recurrence was observed at 1.5 months after the end of therapy. Although the patient was then treated with palliative radiotherapy and SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) chemotherapy, relapses occurred. While the patient was being prepared for autologous stem cell transplantation, he died due to rapidly onset myelosuppression and systemic infections.
DISCUSSION
BPDCN usually presents with cutaneous lesions as nodules, bruise-like patches, and disseminated skin lesions. There is no correlation between clinical presentation and prognosis depending on clinical experiences of a large number of study population [2]. The first presentation is usually one or two isolated cutaneous nodules. In the present case, the initial presentation of BPDCN was a rapidly evolving tumoral mass. Disseminated lesions developed rapidly in the disease course.
BPDCN is a dermal neoplasm spearing epidermis with a grenz zone, frequently infiltrating into subcutaneous fat tissue [3]. BPDCN is consisted of small to medium sized cells with irregular nuclear contours. The typical feature of these cells is having positive cell surface markers for CD4, CD56, CD123 and T cell leukemia/lymphoma 1 (TCL1). Only a few CD4- CD56 + cases of BPDCN are reported [4]. Expression of specific antigens for T and B cell lineages, granulocytes, and monocytes is lacking. Depending on these features, neoplastic cells are accepted to originate from pDC. The main differential diagnoses of BPDCN includes myeloid sarcoma/acute myeloid leukemia (myeloid leukemia cutis), T cell lymphoblastic leukemia/lymphoma (T-ALL/LBL), NK cell lymphoma/leukemia, and mature T cell lymphoma/leukaemia [3,5]. In our case, initial and early cutaneous involvement should have been differentiated from myeloid leukemia cutis. Both neoplasms are morphologically indistinguishable and skin is commonly involved [6]. Myeloperoxidase is more specific for myeloid lineage than CD68 positivity. Negative staining for MPO generally helps to rule out myeloid sarcoma as in our case. However, rarely seen MPO negative leukemia cutis is a challenging problem. Cronin et al. suggested that MPO negative cases with negative for CD4, CD56, CD123, or Tcl-1, or positive for only CD56 are myeloid leukemia cutis [7]. In our case, MPO negativity together with CD4 and CD56 positivity drew away the diagnosis of myeloid leukemia cutis. T-ALL/LBL is a neoplasm that includes CD3+, CD2+, and TdT+ cells and clonal T cell receptor rearrangement, which are T cell specific features. Lack of these T cell markers helped us to rule out T-ALL/LBL. NK/T cell lymphoma nasal type expresses CD56; however, angiocentric and angiodestructive growth pattern are not features of BPDCN. Although cutaneous T cell lymphomas resemble BPDCN, negative T cell receptor gene rearrangement supported BPDCN in our case.
In the present case, detailed immunophenotypic analysis provided us to rule out the differential diagnoses and supported the diagnosis of BPDCN in our case. In a recent study, the typical cells of BPDCN were demonstrated to be resting pDC [8]. Since myelodysplasia and acute myeloid leukemia develop in the course of BPDCN, pDC are strongly suggested to belong to the myelomonocytic lineage. Therefore, BPDCN was classified in the category of acute myeloid leukemia and related precursor neoplasms in 2008 World Health Organization Classification of Haematopoietic and Lymphoid Tissue [1,3].
Prognosis in BPDCN is aggressive and poor. Mean survival time is about 12 months. Advanced age and advanced stage are poor prognostic factors [2]. As soon diagnosed by cutaneous findings, involvement of lymph nodes and leukemic dissemination are observed [7]. Initial response to multiagent chemotherapy is very rapid and favorable; however, recurrences develop rapidly, resulting in poor prognosis [9]. In the present case, almost all cutaneous lesions regressed rapidly in the first course of chemotherapy. Unfortunately, new lesions were observed within 2 months after ceasing therapy. These were resistant to chemotherapy and survival time from the diagnosis was 15 months. In the current management of BPDCN, aggressive therapies including allogenic hematopoietic stem cell transplantation are recommended as soon as the diagnosis was made [10].
In conclusion, BPDCN should be in the clinical and histopathological differential diagnosis of rapidly developing tumoral cutaneous lesions. Early diagnosis is important to initiate aggressive treatments in BPDCN, in which skin involvement is very frequent.
REFERENCES
1. Facchetti F, Jones D, Petrella T. Blastic plasmacytoid dendritic cell neoplasm. In: Tumors WHOCo, ed. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp.145-7.
2. Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L, et al. Blastic plasmacytoid dendritic cell neoplasm: Clinical features in 90 patients. Br J Dermatol. 2013;169:579-86.
3. Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: A clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-9.
4. Choi KW, Lee KY, Lee YK, Ku BS, Kim HS, Kim YH, et al. CD4-/CD56+/CD123+ hematodermic neoplasm showing early liver metastasis. Ann Dermatol. 2010;22:186-90.
5. Riaz W, Zhang L, Horna P, Sokol L. Blastic plasmacytoid dendritic cell neoplasm: Update on molecular biology, diagnosis, and therapy. Cancer Control. 2014;21:279-89.
6. Cronin DM, George TI, Reichard KK, Sundram UN. Immunophenotypic analysis of myeloperoxidase-negative leukemia cutis and blastic plasmacytoid dendritic cell neoplasm. Am J Clin Pathol. 2012;137:367-76.
7. Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: Physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
8. Sapienza MR, Fuligni F, Agostinelli C, Tripodo C, Righi S, Laginestra MA, et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia. 2014;28:1606-16.
9. Dalle S, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, et al. Blastic plasmacytoid dendritic cell neoplasm: Is transplantation the treatment of choice? Br J Dermatol. 2010;162:74-9.
10. Roos-Weil D, Dietrich S, Boumendil A, Polge E, Bron D, Carreras E, et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: A retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121:440-6.
Notes
Source of Support: Nil
Conflict of Interest: None declared.

Comments are closed.