De novo anaplastic Kaposi sarcoma in a HIV-negative man: A case report and review of the literature
Slim Charfi1, Hend Chaabane2, Ikbel Chaari2, Rim Kallel1, Hamida Turki2, Tahya Sellami Boudawara1
1Department of Pathology, CHU Habib Bourguiba, Sfax, Tunisie; 2Department of Dermatology, CHU Hedi Chaker, Sfax, Tunisie
Corresponding author: Prof. Slim Charfi, E-mail: charfislim@gmail.com
Submission: 18.09.2016; Acceptance: 24.11.2016
DOI: 10.7241/ourd.20173.85
ABSTRACT
Anaplastic Kaposi sarcoma is a rare variant of Kaposi’s and is typically associated with an agressive clinical course. We report a case of a 67-year-old HIV negative man, presented with multiple, pink nodules on the left ankle and a keratotic lesion of the right heel. Initial histopathological exam concluded to an undifferentiated sarcoma. A second biopsy was performed and concluded to an anaplastic Kaposi sarcoma. Immunohistochemical study was positive for HHV8. Treatment consisted on a tumor excision of all lesions. Our case and the review of the literature highlight the benefit of the non conservative surgical treatment for this aggressive form of Kaposi sarcoma.
Key words: Anaplastic, Kaposi, Prognosis, Pathology
INTRODUCTION
Kaposi sarcoma has the ability to develop into lesions of varying morphologic appearance. Anaplastic variant is the only variant associated with aggressive behaviour [1]. It is important to be able to recognize this variant in order to avoid potential misdiagnosis and improper management of affected patients. We present here a case of anaplastic KS in a HIV negative patient and a review of literature about anaplastic KS.
CASE REPORT
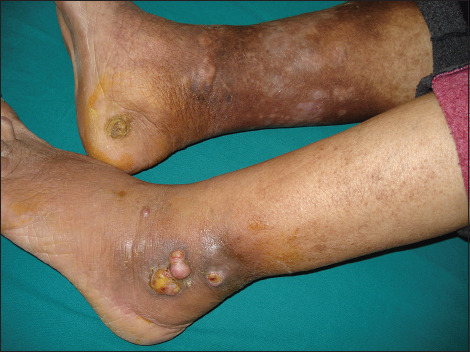
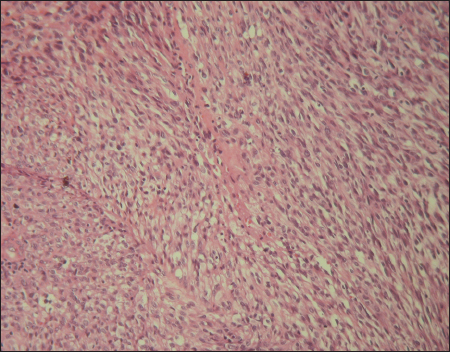
A 67-year-old man with a history of type 2 diabetes, high blood pressure and deep vein thombosis of the left lower leg presented with multiple 0.5-2 cm, pink nodules, located on the left ankle. On the right heel, we noted akeratotic nodular lesion (Fig. 1). All these lesions had a 2 months history. No lesions were noted in the oral mucosa. Laboratory test showed a biological inflammatory syndrome. HIV serology was negative. Clinical impression was that of Kaposi sarcoma and a punch biopsy of lesions was performed. Pathological report, peformed outside of our department concluded to an undifferentiated sarcoma. A second excisional biopsy was performed. Histopathological exam revealed an epidermal ulceration and dermal proliferation of spindle and epithelioid cells. Tumor cells are arranged in fascicles and solid sheets (Fig. 2). Irregular vascular spaces, suggestive of a vasoformative tumor, were focally noted. There are areas of necrosis. The nuclei were very pleomorphic with variably prominent nucleoli. Numerous mitosis were present (10 mitosis per 10 HPF). The stromal component included numerous plasma cells. Immunohistochemical study showed positivity for CD34, CD31 and HHV8 (Fig. 3). The diagnosis of anaplastic KS was made. Additional clinical workup including chest radiography, abdominal ultrasound and computed tomography scan of the chest, abdomen and pelvis were normal. Upper digestive endoscopy was not performed. Treatment has consisted on a complete electrosurgical shave tumor excision. At 13 months of follow-up, the patient was readmitted to our department for a disease progression with a fungiform exophytic mass of the left ankle (Fig. 4). No distant metastatic recurrence was noted. A chemotherapy treatment was instaured.
DISCUSSION
Anaplastic KS was first described in 1959 by Cox and Helwig as KS with increased in the number of mitotic figures and marked cellular pleomorphism [2]. Anaplastic KS is an uncommon histologic variant, representing 0.4% to 2.7% of all the patients affected by KS [3–5]. Clinical data regarding all published cases of anaplastic KS with confirmed HHV8 association are detailed in Table 1. Anaplastic KS arises in patients with a history of KS, or de novo. Factors that predispose to anaplastic progression of KS are not well known [3]. Potential inducers, such as a long course of the disease, lymphedema, chemotherapy and immune system defects in HIV patients are reported [4]. At the best of our knowledge only 14 de novo Anaplasic KS were published [3,5–8]. Nine of these cases were reported by Ramdial et al. [5]. Anaplastic histology has ben described in the context of classic, africain and AIDS-related KS [1]. Anaplastic KS appear to have a predilection for acral location [3–5,9,10]. Only three HIV negative patients with de novo anaplastic KS, as our case, were reported [3,8].
Clinical presentation of anaplastic KS is not specific; they present as pink to purple or reddish cutaneous nodules and/or plaques [4,7]. Some authors reported nodular areas growing rapidly to form a confluent fungiform exophytic mass that was in part ulcerated and necrotic [3,5]. Infiltration of the muscle or bone was described [4]. Salameire et al. report a case of 50-year-old woman who presented with several nodules on lymphedematous arm, 2 yeras after a left mastectomy and a homolateral lymphadenectomy, mimicking a stewart-treves syndrome [8].
Anaplastic KS dispalys a significantly greater degree of nuclear and cellular pleomorphism than conventional nodular KS [1,11]. In addition, there is an increased mitotic activity (mitotic figures range from 10 to 20 per 10 hpf) and atypical mitosis may be encountered (radu, grayson). Necrosis is occasionally noted [9]. The stromal component, like that of classical KS and as reported in our case is plasma cell-rich and contains extravasated erythrocytes [7]. The vasoformative nature of anaplastic KS is not readly apparent. As in our report, Ramdial et al. reported three cases of anaplastic KS with initial erronous diagnosis (malignant peripheral nerve sheath tumor in two cases and metastatic melanoma in one case). In fact, many other malignant spindle cell neoplasms might be entertained in the histologic differential diagnosis of anaplastic KS. It includes certain sarcomas (leiomyosarcoma, spindle cell rhabdomyosarcoma, malignant peripheral nerve sheath tumor, fibrosarcoma), amelanotic spindle cell melanoma, especially when present on acral sites andspindle cell carcinoma [5]. Angiosarcoma must also be considered, particularly if erythrocytes are identified between the markedly atypical spindled cells [9]. A panel of immunohistochemical stains is often required to confirm the diagnosis of anaplastic KS. This panel must include HHV8. Immunostaining for S100 protein can lead to diagnostic pitfalls; in fact Ramdial et al demonstrates the presence of S100 positive cells corresponding to langerhans cells in anapalstic KS [5].
Anaplastic KS is clinically associated with high local aggressiveness, deep invasion and increased metastatic behavior [1,3,7]. Considering our case and those described in the literature, we observe that among 28 patients with anaplastic KS, 9 died of anaplastic KS, 7 died of other causes (including four HIV positive patients) and 2 were alive with anaplastic KS [3–5,7–10,12]. Satta et al. supported an intrinsic genetic instability of the malignant cell resulting in clonal progression of the neoplastic phenotype [3]. Craddock et al. reports that anaplastic portion of the KS respond well to paclitaxel and no liposomal doxorubicin, while the background typical KS lesions have had the opposite behaviour with these two therapies [9]. In the report of Salameire et al, a total regression of the lesion was obtained by elastic contention and intradermic liposomal doxorubicin [8]. Tourlaki et al recommend an aggressive approch, more specifically a non-conservative surgical treatment associated with systemic chemotherapy. Amputation due to deep tissue invasion may be required [4]. Our patient treated with only tumor excision, a frankly disease progression was noted at 13 months of diagnosis.
Anaplastic variant of KS is a poorly recognized associated to an aggressive clinical course and poor prognosis. Histopathological differential diagnoses include numerous undifferentiated high grade malignancies. Non conservative surgical treatment seems appropriate for these patients.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
2. Cox FH, Helwig EB. Kaposi’s sarcoma. Cancer. 1959;12:289-98.
3. Satta R, Cossu S, Massarelli G, Cottoni F. Anaplastic transformation of classic Kaposi’s sarcoma: clinicopathological study of five cases. Br J Dermatol. 2001;145:847-9.
4. Tourlaki A, Recalcati S, Boneschi V, Gaiani F, Colombo A, Mancuso R, et al. Anaplastic Kaposi’s sarcoma: a study of eight patients. Eur J Dermatol. 2013;23:382-6.
5. Ramdial PK, Sing Y, Naicker S, Calonje E, Sewram V, Singh B. Langerhans cells in anaplastic Kaposi sarcoma with a paucivascular phenotype: a potential diagnostic pitfall. Pathol Int. 2011;61:221-7.
6. Smith KJ, Skelton HG 3rd, James WD, Barrett TL, Anderson DW, Angritt P. Angiosarcoma arising in Kaposi’s sarcoma (pleomorphic Kaposi’s sarcoma) in a patient with human immunodeficiency virus disease. Armed Forces Retroviral Research Group. J Am Acad Dermatol. 1991;24:790-2.
7. Yu Y, Demierre MF, Mahalingam M. Anaplastic Kaposi’s sarcoma: an uncommonhistologic phenotype with an aggressive clinical course. J Cutan Pathol. 2010;37:1088-91.
8. Salameire D, Templier I, Charles J, Pinel N, Morand P, Leccia MT, et al. An “anaplastic” Kaposi’s sarcoma mimicking a Stewart-Treves syndrome. A case report and a review of literature. Am J Dermatopathol. 2008;30:265-8.
9. Craddock KJ, Labonte S, Ghazarian D. Anaplastic Kaposi sarcoma resembling epithelioid angiosarcoma in an HIV-positive man. Eur J Dermatol. 2008;18:358-9.
10. Gambassi G, Semeraro R, Suma V, Sebastio A, Incalzi RA. Behavior of Classical Kaposi’s Sarcoma and Coexistence With Angiosarcoma. J Gerontol A Biol Sci Med. 2005;4:520-3
11. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-94.
12. Cerimele D, Carlesimo F, Fadda G, Rotoli M, Cavalieri S. Anaplastic progression of classic Kaposi’s sarcoma. Dermatology. 1997;194:287-9.
Notes
Source of Support: Nil



Comments are closed.