Efficacy and tolerance of 2,3-diphenylcyclopropenone in propylene glycol versus 2,3-diphenylcyclopropenone in isopropanol – a novel formula designed for the treatment of alopecia areata
Tomasz Wasyłyszyn1, Katarzyna Borowska2
1Department of Dermatology, Military Institute of Medicine in Warsaw, Warsaw, Poland; 2Department of Histology and Embryology with Experimental Cytology Unit, Medical University of Lublin, Lublin, Poland
Corresponding author: Prof. Katarzyna Borowska, MD, PhD., E-mail: k_borowska@wp.pl
Submission: 30.11.2016; Acceptance: 11.12.2016
DOI: 10.7241/ourd.20171.04
ABSTRACT
Introduction: Topical immunotherapy of alopecia areata (AA) with contact allergens including 2,3-diphenylcyclopropenone (DCP) have been used for few decades.
Aim: The study introduces a new preparation of DCP in isopropanol for the purpose of the treatment of AA. The aim of the study was to compare new formula with the previously used DCP in propylene glycol. Two groups of twenty patients each, treated with new and old formula respectively were observed for one year. Treatment efficacy and tolerance was then measured and compared.
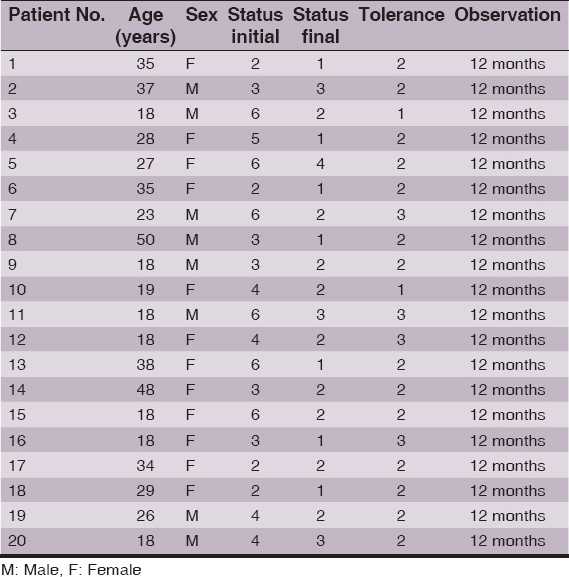
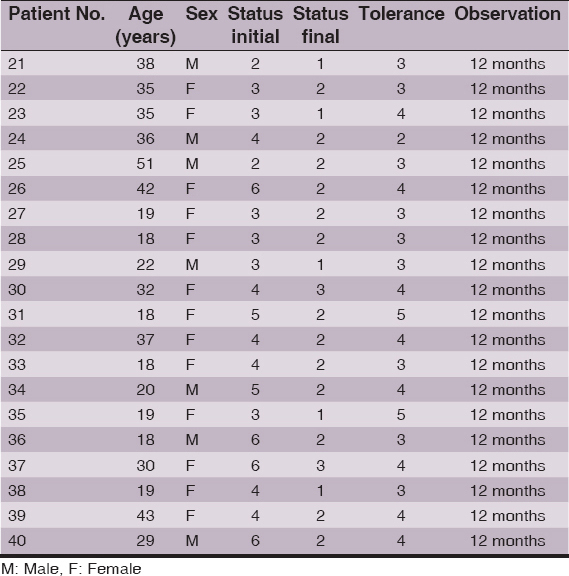
Result: All but one patient responded to the study with partial or complete hair regrowth. Results show improvement after the treatment in both groups with 75% and 85% of patients with cosmetically acceptable hair amount (grades 1 and 2 in the hair loss scale) in DCP in isopropanole and DCP in propylene glycol groups respectively. Complete hair regrowth was obtained in 35% and 25% of patients respectively. Differences in treatment’s efficacy between groups were not statistically important (p = 0.95). Tolerance was generally good but significantly better (p= 0.17) in a group treated with DCP in isopropanol.
Discussion: DCP solutions in isopropanol may have potential in the treatment of alopecia areata. Treatment efficacy in both groups (DCP in propylene glycol and DCP in isopropanol) was the same. Tolerance of the DCP in isopropanol was significantly better than DCP in propylene glycol. Despite better tolerance of DCP in isopropanol during the proper treatment, the sensitization with 3% DCP should be done with the solution in propylene glycol.
Key words: Alopecia areata; 2,3-diphenylcyclopropenone; DCP, Isopropanol; Propylene glycol
INTRODUCTION
Topical immunotherapy of alopecia areata (AA) with contact allergens including 2,3-diphenylcyclopropenone (DCP) have been used for few decades [1–4]. Numerous authors report different data regarding this treatment efficacy; however, it ranges between 20 – 70% of patients with cosmetically acceptable hair regrowth [5,6]. The treatment itself is still in an experimental stage as there is no DCP being available as a brand medication so far. The main reasons for that, besides the preparations being relatively unstable and thus improper for pharmaceutical trade, are the adverse allergic reactions that eventually may lead to the complications [7]. In authors’ experience these reactions do not provoke serious adverse effects if the procedure is managed properly, especially when areas near eyes, ears and neck are avoided during the treatment [5,8,9]. Nevertheless, all the patients report mild to moderate discomfort related to DCP treatment. It includes: itching, erythema, redness and swelling of occipital lymph nodes. Recently authors have analyzed the stability of DCP solutions in various solvents and how addition of water to these solutions have affected their stability [10,11]. Results led to the conclusion that beside propylene glycol, an isopropyl alcohol (isopropanol) is promising solvent for the DCP. Except for the stability, it gives the solutions a proper consistency. Meanwhile, the study revealed that the DCP solutions with acetone, which is widely used as a solvent, are stable only for few days, especially at room temperature [10]. Moreover, all the solutions turned out to be stable only in the refrigerator at temperature of 4 °C irrespective of the solvent used.
Considering the findings mentioned above authors decided to investigate the therapeutic potential and the tolerance to DCP solutions in isopropanol and compare it to the effect obtained during the treatment with DCP in propylene glycol.
METHODS
Clinical and demographic data of the patients are summed in Tables 1 and 2. After having an agreement with ethics committee, authors arranged two groups of AA patients, 20 patients in each group. Groups were randomized so that the mean initial status measured by own scale (described below) was equal in each group – thus meaning that groups had similar mean hair loss at the beginning of the study. Solutions were made of 98% diphenylcyclopropenone chemical by Sigma Aldrich (No. 177377). Basic solutions of 3% DCP were prepared in propylene glycol and isopropanol. All solutions were kept in the refrigerator at the temperature of 4 °C according to previously collected data. From the initial solutions, the solutions of 0.5%, 0.3%, 0.2%, 0.1% and 0.05% were made in each solvent in order to perform the proper treatment.
In both groups (the DCP in propylene glycol and DCP in isopropanol), on the first visit, 3% DCP was applied to bald areas on the top of the scalp to obtain sensitization. Areas near the neck and ears were avoided to prevent irritation – even if there were bald patches. Patients were instructed to avoid situations which may lead to sweating (like sports) for the next two days. During 24 to 48 hours after application, a reaction including itching, redness, edema and the presence of vesicles with serous liquid occurred. After 2 days, patients were told to wash their scalps and the reaction faded. Further applications took place during 1 week intervals. The DCP concentration was at that time between 0.05% and 0.5% (usually about 0.1%), depending on the intensity of the reaction after sensitization. Patients were equipped with the tube of strong corticosteroid ointment (clobetacole propionate 0.05%) and were advised to use it if allergic reaction to DCP occurs on the body parts. All the patients were observed for 12 months.
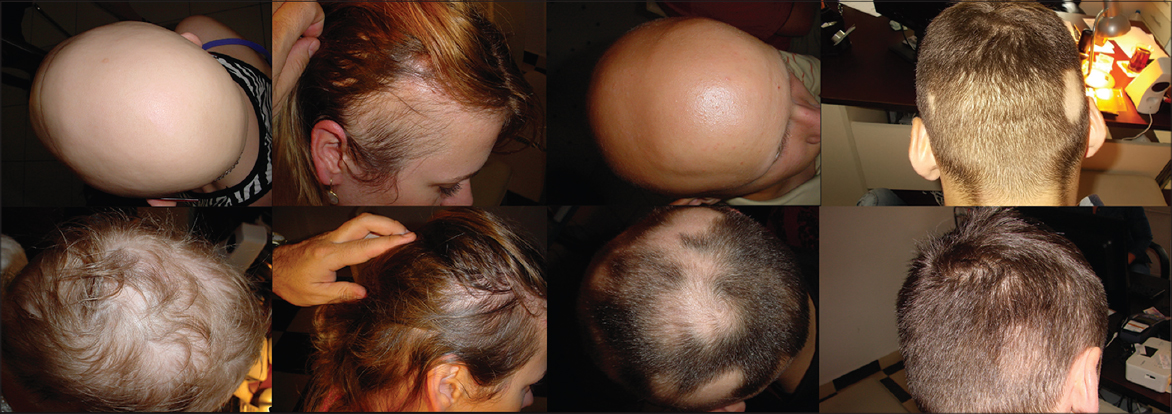
To assess the grade of hair loss or hair regrowth authors used own invented scale that was used before [5,8,9]. Grade 1 of this scale indicated no hair loss or complete hair regrowth, grade 2 showed bald area below 20% of the scalp surface, grade 3- represented bald area between 21 and 50% of the scalp surface, grade 4 – had a bald area between 51 and 80% of the scalp area, grade 5 – reflected a bald area between 81 and 99% of the scalp surface and finally, grade 6 – total 100% hair loss (alopecia totalis). In the discussion grades 1 and 2 are considered as “cosmetically acceptable”. The assessment was made twice: at the beginning and at the end of the 12 months of observation.
At the end of the study each patient filled the questionnaire regarding subjective tolerance of the treatment. The tolerance was measured in six grade scale in which grade 1 indicated perfect tolerance and no side effects and grade 6 meant that treatment was intolerable and had to be withdrawn. Details of the tolerance scale are listed in the Table 3.
Statistical Analysis
For the purpose of this study Kruskal-Wallis rank sum test, Bartlett test of homogeneity of variances and Shapiro-Wilk normality test were used.
RESULTS
All but one patient responded to the study with partial or complete hair regrowth. Results show improvement after the treatment in both groups with 75% and 85% of patients with cosmetically acceptable hair amount (grades 1 and 2 in the hair loss scale) in DCP in isopropanole and DCP in propylene glycol groups respectively. By comparison initial values of “cosmetically acceptable” hair in these groups were 20% and 10% respectively. Complete hair regrowth was obtained in 35% and 25% of patients respectively (initial amount of complete regrowth was zero obviously). Differences in treatment’s efficacy (Fig. 1) between groups were not statistically important (p = 0.95).
Tolerance was generally good but significantly better (p= 0.17) in a group treated with DCP in isopropanol (Fig. 2). While patients treated with DCP in propylene glycol usually reported tolerance level between 3 and 4 (Table 3), those treated with DCP in isopropanol reported tolerance grade 1 to 2. However, in the latter group allergic reactions affecting the body were reported more often; usually disappearing during one day but annoying. The itching patches on the upper body parts required the usage of corticosteroid ointment for few days. None of the patients had to withdraw the treatment because of the side effects.
DISCUSSION
Results are similar to the ones that authors obtained in previous studies [5,8]; however, it is worth mentioning that observation in the present study lasted one year versus two years in previous ones. Hence the result after another year of observation may vary in every way. Typically patients showed improvement but required further treatment (Fig. 3). What is important, conclusion of this study is that tolerance of DCP dissolved in isopropanol is to be better than previously used DCP in propylene glycol. It is patients’ subjective impression that make them continue or withdraw the treatment. With the exception of sensitization with high concentration 3% DCP most patients treated with DCP in isopropanole described side effects as “none” or “mild that do not disturb routines”. In contrast, in the DCP in propylene glycol group patients complained of “side effects that affect routines”. None of them had to stop the treatment; however, a one – two week break in a treatment routine was sometimes necessary. At the beginning of their experiences with DCP authors noticed that preparations of DCP that were older than 6 months had different clinical effect on the patients scalp. While clinical efficacy seemed to gradually fade with the solution’s age the irritation was getting bigger. Now it is known that DCP solutions kept at room temperature decompose slowly and the product of DCP decomposition – diphenylacetylene (DPA) seems to have irritating qualities. Although pharmacokinetics of DCP on the surface of the skin is unknown, recent studies [10,11] confirm following hypothesis. Both solvents: isopropanol and propylene glycol are perfect solvents for DCP while solutions are kept in the refrigerator. The concentration of DCP against it’s main decomposition product – diphenylacetylene (DPA) remains unchanged after 60 days of measurement and probably longer in propylene glycol at temperature of 4 °C [11]. The situation changes when solutions of DCP are stored at room temperature or higher and are contaminated with water. Large amount of water or NaCl 0.9% solution accelerates DCP’s decomposition [10]. Propylene glycol creates dense, sticky layer on the surface of the skin and droplets of sweat appear quickly underneath this layer, especially during sunny days according to authors’ observations. This probably leads to DPA aggregation and eventually to bigger irritation. In contrast, isopropanol evaporates quickly just seconds after it is applied to the surface of the scalp. If the patient avoids situation that might lead to sweating as he was instructed, a thin layer of DCP not contaminated with DPA will remain on his scalp until washing out. It causes usually well tolerable and controllable allergic reaction necessary to obtain therapeutic effect but not the toxic reaction leading to discomfort. The phenomenon that higher DCP concentration in isopropanole used during sensitization in our study led to irritation on the body parts can also be explained. In this special situation the amount of DCP on patient’s scalp is 30 times bigger than in 0.1% solution usually used during proper treatment. Authors believe that sensitization with 3% DCP is the only situation when properties of propylene glycol, especially its “stickiness”, make it more suitable, as it is more unlikely for DCP from patient’s scalp to be spread all over the body during nightime.
CONCLUSIONS
- DCP solutions in isopropanol may have potential in the treatment of alopecia areata.
- Treatment efficacy in both groups (DCP in propylene glycol and DCP in isopropanol) was the same.
- Tolerance of the DCP in isopropanol was significantly better than DCP in propylene glycol.
- Despite better tolerance of DCP in isopropanol during the proper treatment, the sensitization with 3% DCP should be done with the solution in propylene glycol.
REFERENCES
1. Happle R, Klein HM, Macher E, Topical immunotherapy changes the composition of the peribulbar infiltrate in alopecia areataArch Dermatol Res 1986; 278: 214-8.
2. Hoting E, Boehm A, Therapy of alopecia areata with diphencyproneBr J Dermatol 1992; 127: 625-9.
3. Borowska K, Wasyłyszyn T, Zastosowanie lecznicze alergenu kontaktowego difenylocyklopropenonu. (Medical applications for the contact allergen – diphenylcyclopropenone.)Int Rev Allergol Clin Immunol Fam Med 2016; 22: 173-8.
4. Borowska K, Wasyłyszyn T, Procesy immunologiczne zachodzące w łysieniu plackowatym. (Immunological processes in alopecia areata.)Int Rev Allergol Clin Immunol Fam Med 2016; 22: 48-52.
5. Wasyłyszyn T, Kozłowski W, Zabielski SL, Changes in distribution pattern of CD8 lymphocytes in the scalp in alopecia areata during treatment with diphencyproneArch Dermatol Res 2007; 299: 231-7.
6. Chiang K, Atanaskova Mesinkovska N, Amoretti A, Piliang MP, Kyei A, Bergfeld WF, Clinical efficacy of diphenylcyclopropenone in alopecia areata:retrospective data analysis of 50 patientsJ Am Acad Dermatol 2014; 71: 595-7.
7. Culp BL, Wells MJ, Generalized urticaria with use of diphencyprone in the treatment of wartsJ Drugs Dermatol 2007; 6: 529-30.
8. Wasyłyszyn T, Borowska K, Possible advantage of imiquimod and diphenylcyclopropenone combined treatment versus diphenylcyclopropenone alone:An observational study of nonresponder patients with alopecia areataAustralas J Dermatol 2016; 17: 1-5.
9. Borowska K, Wasyłyszyn T, Zabielski S, The efficacy and safety of topical therapy with diphenylcyclopropenone in the treatment of alopecia areata – a retrospective study. (Ocena efektywności i bezpieczeństwa difenylcyklopropenonu w leczeniu miejscowym łysienia plackowatego – badania retrospektywneInt Rev Allergol Clin Immunol Fam Med 2016; 22: 27-32.
10. Borowska K, Wasyłyszyn T, Studies on stability of 2,3-diphenylcyclopropenone in contact with water and aqueous NaCl solutions. Conclusions for the purpose of topical therapy of patients with alopecia areataActa Pol Pharmaceut 2016 in press; 73: 6
11. Wasyłyszyn T, Borowska K, The stability of solutions of 2,3-diphenylcyclopropenone in various solvents. A novel formula – diphenylcyclopropenone in isopropanol may be useful in topical therapy of patients with alopecia areataActa Pol Pharmaceut 2017 in press; 74: 2
Notes
Source of Support: Nil
Conflict of Interest: None declared.

Comments are closed.