Solitary eccrine syringofibroadenoma: a case report showing papillary tubular adenoma-like features
Toshiyuki Yamamoto1, Keiko Miura2, Hiroo Yokozeki1
1Department of Dermatology, Tokyo Medical and Dental University, 1-5-45 Yushima, Tokyo 113-8519, Japan, 2Department of Pathology, Tokyo Medical and Dental University, 1-5-45 Yushima, Tokyo 113-8519, Japan
ABSTRACT
We herein describe a case showing eccrine syringofibroadenoma occurred on the dorsum of the right foot of a 46-year-old Japanese female. Histopathologic examination revealed anastomosing cords and strands of cuboidal epithelial cells extending from the epidermis to the upper dermis, with a number of well-defined ducts suggesting eccrine ductal origin. In addition, there were papillary tubular adenoma-like ductal structures lined by a few rows of epithelial cells with papillary projections into the lumen surrounded by fibrous stroma in the mid-dermis. It is of note that various histologic features showing different differentiation were seen in a single lesion of eccrine syringofibroadenoma.
Key words: Eccrine syringofibroadenoma; Papillary tubular adenoma; Foot
INTRODUCTION
Eccrine syringofibroadenoma (ESFA) is a rare tumor originated from eccrine ductal portion [1,2]. Clinically, ESFA presents a solitary papule, nodule or plaque to multiple lesions. Cases with multiple papular or macular lesions have been often associated with hereditary ectodermal dysplasia [3]. Recent findings have classified ESFA into several groups including, solitary ESFA, multiple ESFA with hydrotic ectodermal dysplasia, multiple ESFA without associated cutaneous findings, nonfamilial unilateral linear ESFA, and reactive ESFA associated with inflammatory or neoplastic dermatoses [4]. We report a case of solitary ESFA which histologically showed various degrees for sweat gland differentiation.
CASE REPORT
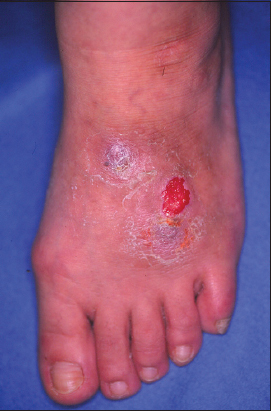
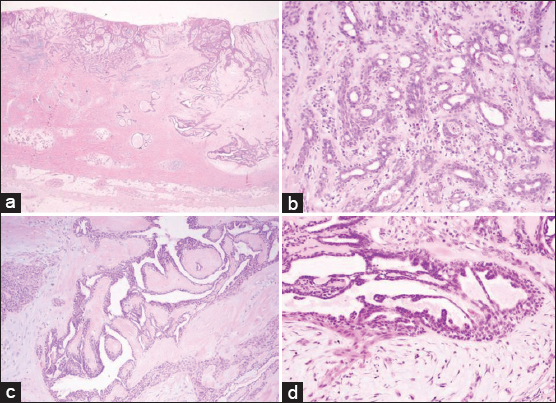
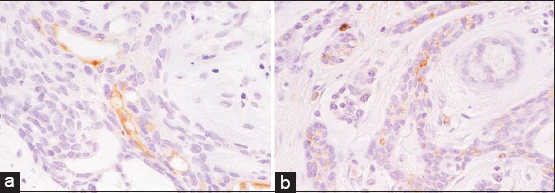
A 46-year-old Japanese woman presented with a solitary, 2.3×2.0 cm tumor involving the dorsum of her left foot over 10 years previously. The tumor was erosive, red-colored, and elastic hard (Fig. 1). She, as well as family members, did not have histories of ectodermal dysplasia. Laboratory examination denied diabetes mellitus. Histological examination of the totally resected tumor showed the anastomosing strands of cuboidal cells extending from the epidermis to the upper dermis (Fig. 2a). In addition, syringoma-like dilated eccrine sweat duct proliferation was also observed in the mid-dermis (Fig. 2a). The epithelial cords contained ductal structures lined by a few layers of flattened cells. Well-defines ductal structures were abundant with the proliferative cord cells (Fig. 2b). Furthermore, ductal structures were found in the mid-dermis surrounded by dense and hyalinized stroma (Fig. 2c), which were lined by two or more rows of epithelial cells that partially form papillary projections into the lumen (Fig. 2d). There was an inflammatory infiltrate of mononuclear cells in the upper dermis with an increased vascularity. Immunohistochemical study was performed by a standard avidin-biotin peroxidase technique. Formalin-fixed, paraffin-embedded sections were prepared on poly-L-lysin coated slides, dewaxed, rehydrated and rinced with phosphate buffered saline (PBS). Endogenous peroxidase activity was blocked by treatment with 0.3 % H2O2 in methanol for 15 min. Sections were then incubated with the primary antibodies against S-100 and carcinoembryonic antigen (CEA) (Dakopatts, Dako Japan, Tokyo) at room temperature, followed by incubation with biotin-labeled secondary antibody, and finally avidin-biotin-peroxidase complex. Sections were developed with 3,3-diaminobenzidine solution, dehydrated, cleared and amounted. Negative controls were prepared with omitting of primary antibodies and by the substitution by isotype-matched control IgG. CEA was detected on the tumor cells of ductal wall (Fig. 3a). S-100 was detected on the cuboidal cells, whereas negative in the ductal structure of ESFA (Fig. 3b). The neoplastic cells were negative with monoclonal antibodies to desmin and vimentin.
DISCUSSION
ESFA have been reported to occur in association with inflammatory disorders such as bullous pemphigoid [5], erosive palmoplantar lichen planus [6], lepromatous leprosy [7], and diabetes mellitus [8,9]. In addition, reactive ESFA has also been reported to occur subsequently on the hyperkeratotic palms and soles [10–15]. Our patient had histories of neither those systemic diseases nor ectodermal dysplasia, but the nodule existed on the dorsum of the foot for long time, suggesting that the tumor was reactive to mechanical stimuli rather than the true neoplasm. In our case, it is of interest that papillary tubular adenoma-like differentiation was partially noted as well as typical ESFA. Papillary tubular adenoma was first described by Abenoza and Ackerman that includes both papillary eccrine adenoma and tubular apocrine adenoma [16]. The histological features are characterized by a relatively well-circumscribed dermal tumor containing numerous tubular and cystic structures with intradermal papillary projections surrounded by fibrous stroma. Thus far, more than 50 cases of ESFA have been reported, however, to our knowledge, ESFA showing papillary tubular adenoma-like lesions has not been reported. Although ESFA is an eccrine tumor, the presence of papillary projections and decapitation-like findings seen in our case favor apocrine lesions. Ishiko et al. [17] also reported the close association of both eccrine and apocrine tumor in a single tumor. On the other hand, two histopathological variants were noted in separate portion of ESFA occurred in one patient [18]. Moreover, syringoma-like dilated sweat duct was seen in the mid-dermis in our case. Sweat gland proliferation has been previously reported in a variety of inflammatory skin diseases, as well as benign and malignant neoplasms [19]. Hara et al. [20] also reported syringoma-like structures intimately associated with epithelial cords in ESFA. Thus, syringoid ductal proliferation seems not to be rare. In this patient, ESFA occurred on the dorsum of the foot which is susceptible for chronic, recurrent stimuli. Since ESFA is suggested to occur reactively in response to mechanical stimuli, we speculate that various differentiation was also induced during repair process.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Mascaro JM, Considerations sur les tumeurs fibro-epitheliales: le syringofibroadenoma eccrineAnn Dermatol Syphiligr 1963; 90: 143-53.
2. Lui H, Stewart WD, English JC, Wood WS, Eccrine syringofibroadenomatosis: a clinical and histologic study and review of the literatureJ Am Acad Dermatol 1992; 26: 805-13.
3. Hurt MA, Igra-Serfaty H, Stevens CS, Eccrine syringofibroadenoma (Mascaro): an acrosyringeal hamartomaArch Dermatol 1990; 126: 945-9.
4. French LE, Reactive eccrine syringofibroadenoma: an emerging subtypeDermatology 1997; 195: 309-10.
5. Nomura K, Hashimoto I, Eccrine syringofibroadenomatosis in gtwo patients with bullous pemphigoidDermatology 1997; 195: 395-8.
6. French LE, Masgrau E, Chavaz P, Saurat J-H, Eccrine syringofibroadenoma in a patient with erosive palmoplantar lichen planusDermatology 1997; 195: 399-401.
7. Mehregan AH, Marufi M, Medenica M, Eccrine syringofibroadenoma (Mascaro): report of two casesJ Am Acad Dermatol 1985; 113: 433-6.
8. Gambini C, Rongioletti F, Semino MT, Rebora A, Solitary eccrine syringofibroadenoma (or eccrine syringofibroadenomatous hyperplasia?) and diabetic polyneuropathyDermatology 1996; 193: 68-9.
9. Utani A, Yabunami H, Kakuta T, Endo H, Shinkai H, Reactive eccrine syringofibroadenoma: an association with chronic foot ulcer in a patient with diabetes mellitusJ Am Acad Dermatol 1990; 41: 650-1.
10. Starink TM, Eccrine syringofibroadenoma: multiple lesions representing a new cutaneous marker of the Schopf syndrome, and solitary non-hereditary tumorsJ Am Acad Dermatol 1997; 36: 569-76.
11. Alli N, Polat M, Çinar SL, Kulaçoglu S, Eccrine syringofibroadenomaEur J Dermatol 2008; 18: 478-9.
12. Singh N, Chandrashekar L, Shakthi P, Thappa DM, Badhe BA, Sylvia MT, Reactive eccrine syringofibroadenomatosis presenting as bilateral plantar hyperkeratosisIndian J Dermatol 2015; 60: 403-5.
13. Tey HL, Characterizing the nature of eccrine syringofibroadenoma: illustration with a case showing spontaneous involutionClin Exp Dermatol 2009; 34: e66-8.
14. Temnithikul B, Jerasutus S, Sudtikoonaseth P, Voravutinon N, Kootiratrakarn T, Kattipathananpong P, Eccrine syringofibroadenoma (ESFA): a report of two casesDermatol Pract Concept 2016; 6: 5-8.
15. Bandyopadhyay D, Chattopadhyay S, Saha S, Reactive eccrine syringofibroadenoma on a leprous footIndian J Dermatol Venereol Leprol 2015; 81: 64-6.
16. Abenoza P, Ackerman AB, Papillary tubular adenomas, in: Neoplasms with Eccrine Differentiation 1st ed. Lea&Febgier; Philadelphia, London: 1990; 353-70.
17. Ishiko A, Shimizu H, Inamoto N, Nakamura K, Is tubular apocrine adenoma a distinct clinical entity?Am J Dermatopathol 1993; 15: 482-7.
18. Van Leeuwen RL, Lavrijsen APM, Starink TM, Eccrine syringofibroadenoma: the simultaneous occurrence of two histopathological variants (conventional and clear-cell type) in one patientBr J Dermatol 1999; 141: 947-9.
19. Mehregan AH, Proliferation of sweat ducts in certain diseases of the skinAm J Dermatopathol 1981; 3: 27-31.
20. Hara K, Mizuno E, Nitta Y, Ikeya T, Acrosyringeal adenomatosis (eccrine syringofibroadenoma of Mascaro): a case report and review of the literatureAm J Dermatopathol 1992; 14: 328-39.
Notes
Source of Support: Nil
Conflict of Interest: None declared.
Comments are closed.