|
Get Citation
|
|
|
Lenz CR, Middleton KA. Cutaneous metastasis heralding invasive ductal carcinoma of the breast in a 33 year old patient. Our Dermatol Online. 2016;7(4):415-418. |
|
|
Download citation file:
|
Cutaneous metastasis heralding invasive ductal carcinoma of the breast in a 33 year old patient
Crystal R. Lenz, Kirk A. Middleton
18th Medical Group, Kadena Air Base, Japan
ABSTRACT
Cutaneous metastatic carcinoma is an unusual clinical finding, with an estimated overall incidence of 5.3%. While it is more frequently found in association with breast cancer than visceral malignancies, it is rarely identified until late in the course of the disease, and quite uncommonly as the heralding manifestation of the underlying malignancy. Because of the prevalence in breast cancer, it is important for primary care clinicians and dermatology specialists alike to be aware of this potential presentation, and able to identify such cutaneous manifestations both in the post breast reconstruction patient as well as the rare patient in which cutaneous manifestations arise as the first indication of the underlying malignancy. This case presentation is an example of the latter situation, with infiltrating ductal carcinoma (IDC) of the breast presenting as a small erythematous skin nodule in a young healthy patient with no known risk factors for breast cancer and no initial radiographic abnormalities to indicate presence of the underlying malignancy at the time of dermatologic evaluation. A review of the relevant literature is included to highlight key concepts.
Key words: Breast cancer; Cutaneous metastases; Invasive ductal carcinoma
INTRODUCTION
Breast cancer is the second most common malignancy among women (after non-melanoma skin cancer), with a lifetime risk of 13% [1,2]. Breast cancer is also the malignancy in which cutaneous metastases are most likely to manifest. While cutaneous metastases is generally regarded as a rare occurrence, with prevalence of 0.7 to 9 percent of all visceral malignancies, the documented incidence in breast cancer is as high as 23.9% [2]. Cutaneous metastasis is also typically seen as a late occurrence in the progression of metastatic disease [3], but there are exceptions to this rule as well, such as the case presented below in which the cutaneous manifestations herald the underlying disease process. Cutaneous lesions may also be the first sign of recurrence after initial treatment of malignancy, which has been more commonly reported in the literature [4]. In all cases, the ability of clinicians to identify cutaneous metastases can result in improved outcomes for patients.
CASE REPORT
Our patient is a healthy 33 year old G2P2 Caucasian female with menarche at age 12 and first live birth at age 27. She used oral contraceptive pills for a total of 12yrs, stopping in 2013 and has no personal or family history of breast cancer. She initially noticed a skin lesion on her right breast in July of 2015, described as a “bump” close to the nipple that she originally thought was a bug bite. On initial exam, approximately 10 days after presentation of the skin lesion, she was found to have a rubbery, freely mobile nodule, less than 1mm in size, located at the 2 o’clock position approximately 4cm from the right nipple. There were no changes to breast size, shape or symmetry and also no nipple discharge or other associated symptoms. A trial of topical mupirocin did not improve the skin lesion and a breast ultrasound combined with diagnostic mammogram showed no radiographic abnormality.
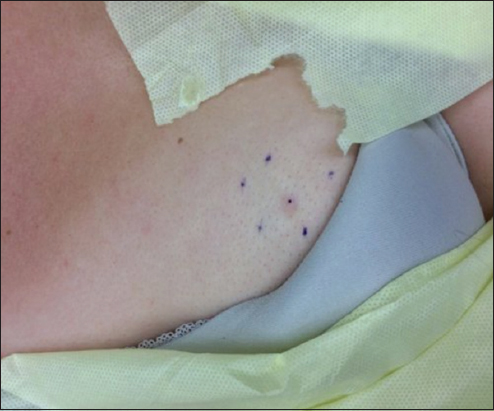
The lesion was re-evaluated by another clinician in October 2015, measured to be approximately 4mm in diameter and described as a pink, raised papule (Fig. 1). A trial of topical clobetasol cream also proved to be of no benefit and, upon evaluation by the local dermatologist in early December 2015, a 4mm punch biopsy was taken for definitive diagnosis. The results of this biopsy showed dermal infiltration of atypical epithelial cells forming small clusters, with presence of glandular structures. The lesion was strongly highlighted with immunohistochemical (IHC) stain for GATA-3 which, in this clinical setting was considered specific for primary carcinoma of the breast. The pathological diagnosis was thus deemed carcinoma, consistent with infiltrating ductal breast carcinoma. It exhibited nuclear grade 2 and was shown to be estrogen and progesterone receptor positive. The patient’s care was quickly transferred to a multi-disciplinary breast cancer center.
At this multi-disciplinary breast cancer center in early January 2016, repeat mammogram and ultrasound of the right breast now showed a 0.5 x 0.7 x 1.0 cm irregular hypoechoic lesion with micro-lobulated and angular margins approximately 1cm below the skin surface at the 2 o’clock position 4cm from the right nipple. The lesion demonstrated multidirectional splitting of cooper’s ligaments to the skin surface and color Doppler of the lesion demonstrated increased vascularity. No other lesions or masses were identified. The following week, an MRI of the breasts offered additional visualization of the right breast mass, centered 8mm deep and 8mm caudal to the visible skin lesion, with an overall dimension of 1.4 x 2.0 x 1.4cm. The mass was noted to have the typical appearance of infiltrating ductal carcinoma, with the skin lesion representing direct extension along Cooper’s ligaments to the overlying dermis.
After discussing options with the surgeon, the patient elected for right simple mastectomy which was completed in mid-January 2016, along with sentinel lymph node biopsy. Intra-operative pathology showed no disease in the sentinel node, but final pathologic evaluation did reveal micro-metastasis and the patient was then taken for a completion axillary dissection. Pathology from the axillary dissection showed reactive changes but no evidence of malignancy. Medical and radiation oncology evaluations in February and early March 2016 assigned staging of IIA (pT1cN1aMx); ER (40%)/PR(70%)+ HER-2 neu negative (IHC 1+); moderately differentiated; oncotypeDx score 22; s/p R simple mastectomy ALND; 1/1 SLNS & ALSND 0/52 LN’s +. Though she did not meet the typical criteria for adjuvant radiotherapy (tumor over 5cm, four or more lymph nodes positive, or positive margins after surgical excision), adjuvant chemotherapy and radiotherapy were recommended based on her younger age as well as the fact that one of her lymph nodes had extracapsular extension and lymphovascular space invasion noted within the primary breast specimen. All of these facts put her at higher risk for local regional recurrence, thus she agreed to adjuvant chemotherapy and radiation treatment.
Dose dense adjuvant Chemotherapy (Doxorubicin and Cylcophosphamide) was initiated in early March 2016. She experienced mild anemia, gastroesophageal reflux and constipation, but otherwise no significant side effects from treatment. She completed her fourth and final cycle in mid-April 2016 and started Taxol treatment in early May, with plans for 28 day cycles for a total of 3 cycles of treatment. At the time of this publication, she remains cancer free and continues to tolerate treatment well.
DISCUSSION
The prevalence of cutaneous metastasis, particularly in breast cancer, warrants attention from primary care clinicians as well as dermatology specialists. Key to recognition is knowing what skin lesion characteristics should raise concern. These metastatic lesions may mimic a variety of benign dermatological conditions such as cutaneous cysts, hemangiomas, herpes zoster eruptions, alopecia, and erysipelas depending on the clinical scenario [5]. In a retrospective analysis of 4,020 patients, Lookingbill et al found nodules to be the most frequent clinical presentation in cutaneous metastases [6]. Another review by Mordenti et al also supported this finding, reporting papules and/or nodules in 80% of patients with cutaneous metastases. They noted telangiectatic carcinomas in 3%, alopecia neoplastica in 2% and zosteriform type in 0.8% of patients [7]. The findings are slightly different in inflammatory breast carcinoma, with erythema noted in 51% of cases, a palpable mass in another 51% of cases, and breast enlargement in 43%. These skin changes started, on average, 10 weeks prior to diagnosis [8]. Nodular metastatic carcinomas can present as firm, solitary or multiple skin masses and may also be accompanied by pigmentation or irregular borders suggestive of melanoma or pigmented basal cell carcinoma [9,10]. Telangiectatic metastatic carcinoma is characterized by papulovesicular lesions with dilated vascularity on an erythematous base which is often associated with intense pruritus. Alopecia neoplastica is believed to be caused by hematogenous spread and manifests as circular areas of alopecia on the scalp which may easily be confused with other types of alopecia (i.e. Areata), but with marked induration [9]. A study out of Turkey evaluating 1,287 cases of cutaneous metastases found that 40% of those individuals with cutaneous metastases also exhibited internal metastases. The cutaneous metastasis was the first sign of malignancy in one case and diagnosed simultaneously in one other patient with breast cancer. The most common sites of cutaneous metastases were the anterior chest wall and lateral trunk [10]. The findings common to all of these studies were that the most common site for cutaneous metastasis is the anterior chest wall, and the most common clinical presentation is multiple papulo-nodular lesions.
There are numerous mechanism by which malignancies may metastasize to the skin. In the Turkish study, cutaneous metastases were noted to be the result of direct invasion in 4 cases, local metastasis in 5 cases, and distant metastasis in 6 cases. All 4 of the direct extensions and one case of local metastasis were breast cancers. The authors also noted that cutaneous metastases showed predilection for certain regions based on the type of primary malignancy, with breast and lung cancers frequently metastasizing to the chest wall and bowel, ovary and bladder cancers metastasizing to the abdominal wall [10]. One additional mechanism for cutaneous metastasis not discussed in the Turkish study is iatrogenic implantation, such as that seen in the case presented by Cho et al in which a 53 year old female with invasive ductal carcinoma presented with a metastatic skin lesion 3yrs after ultrasound guided core needle biopsy at the site. Their investigation supported the recommendation for excision of the needle tract at the time of definitive surgery for invasive breast cancers [11].
Dermal lesions are not uncommon in the breast, and imaging studies are often relied upon to rule out underlying malignancy. As our case demonstrates, breast imaging may not always reveal the underlying malignancy at the time of dermatological presentation. Standard dermal lesions are extra-parenchymal in location, lying anterior to the superficial pectoral fascia in subcutaneous fat and in the echogenic layer of deep dermis on ultrasound. Most of these lesions are benign, and not specific to the breast (such as epidermal inclusion cyst, sebaceous cyst, vascular tumors, lipoma, infections, scars and fat necrosis), making identification of cutaneous metastasis difficult. The nipple areola complex should, however, be given special attention when it comes to evaluation of dermal lesions of the breast, because this complex is unique in that it contains lactiferous ducts which extend from deep mammary glands and are associated with modified sebaceous glands. This causes lesions in this area to be intra-parenchymal in location and subject to development of abnormalities of ductal origin, including carcinoma [12]. There have been several case reports of malignancy (primarily IDC) which appeared as a benign cyst on ultrasound. Because the masses may be indistinguishable on imaging, clinical history is key and there should be a low threshold for biopsy, particularly when there is no obvious sign of infection [13].
Because imaging cannot be relied upon to definitively identify all breast malignancies, biopsy is often necessary when sufficient clinical concern is present. However, even histologic evaluation may prove difficult in some cases, particularly with cutaneous metastasis. It may be difficult to track the differentiation from the primary malignancy, as tumor cells can mimic specific dermal structures. This phenomenon was well demonstrated in the case study presented by Müller et al in which a moderately differentiated IDC of the right breast resulted in cutaneous metastasis (presenting as a reddish, indolent scalp nodule with local alopecia) which mimicked a sebaceous gland neoplasm. In this case, it was primarily the patient’s past medical history of IDC in the left breast 5 years earlier that increased suspicion for breast etiology and eventually lead to the proper diagnosis [14]. Hisaoka et al offer an additional evaluation of the available literature regarding identification of sebaceous carcinoma of the breast, and emphasize the importance of IHC evaluation as a means by which to differentiate the etiology of the abnormal cells [15]. One final publication which demonstrates the role of IHC in assessment of suspicious breast lesions is a case of IDC presenting as a breast abscess, presented by Bhandari et al. This review importantly highlights the fact that invasive cancers demonstrate a lack of both basement membrane and myoepithelial cells, and places emphasis on utilization of the numerous myoepithelial markers which can help identify the invasive nature of the malignancy, with SMM-HC (smooth muscle myosin heavy chain) thought to be the most specific [16].
CONCLUSION
Clinicians should be suspicious of acute-onset, persistent, firm papulo-nodules, especially on the chest. A thorough medical history and physical exam should be accompanied by breast imaging and a low threshold for biopsy of any concerning skin lesions as described above. As this case demonstrates, there are lethal malignancies which, in the early stages, may evade all traditional forms of screening and present solely with cutaneous manifestations. The well trained evaluating clinician with low threshold for biopsy of suspicious lesions will lead to improved clinical outcomes for patients experiencing cutaneous metastases, whether early or late in the course of malignancy.
Acknowledgements
The authors would like to thank Dr. Jason Ballin for his guidance during the evaluation of this patient, as well as his professional review of this article. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force, the Department of Defense, or the U.S. Government.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Krathen RA, Orengo IF, Rosen T, Cutaneous metastasis: A meta analysis of dataSouth Med J 2003; 96: 164-7.
2. Nava G, Greer K, Patterson J, Lin KY, Metastatic cutaneous breast carcinoma: A case report and review of the literatureCan J Plas Surg 2009; 17: 25-7.
3. Santiago F, Saleiro S, Brites MM, Frutuoso C, Figueiredo A, A remarkable case of cutaneous metastatic breast carcinomaDermatol Online J 2009; 15: 10-
4. Pakula A, Recognizing malignant skin changes following breast cancerAm Fam Physician. 992 45: 1287-92.
5. Hussein MR, Skin metastasis: a pathologist’s perspectiveJ Cutan Pathol 2010; 37: e1-20.
6. Lookingbill DP, Spanger N, Helm KF, Cutaneous metastases in patients with monastic carcinoma: A retrospective study of 4020 patientsJ Am Acad Dermatol 1993; 29: 228-36.
7. Mordenti C, Peris K, Concetta Fargnoli M, Cerroni L, Chimenti S, Cutaneous metastatic breast carcinomaActa Dermatovenerol 2000; 9:
8. Haagensen CD, Diseases of the Breast Philadelphia: WB Saunders; 1971; 576-84.
9. Schwartz RA, Cutaneous metastatic diseaseJ Am Acad Dermatol 1995; 33: 161-82.
10. Gül U, Kiliç A, Gönül M, KülcüCakmak S, Erinçkan C, Spectrum of cutaneous metastases in 1287 cases of internal malignancies: A study from TurkeyActa Derm Venereol 2007; 87: 160-2.
11. Cho E, Kim MH, Cha SH, Cho SH, Oh SJ, Lee JD, Breast Cancer Cutaneous Metastasis at Core Needle Biopsy SiteAnn Dermatol 2010; 22: 238-40.
12. Shafqat G, Khan S, Minhas K, Afzal S, Infiltrating ductal carcinoma of breast presenting as areolar dermal lesionJ Coll Physicians Surg Pak 2012; 22: 323-4.
13. Moon WK, Park JM, Kim Yi, Inflammatory and infectious diseases of the breast: imaging findingsPostgraduate Radio.l 2000; 20: 131-242.
14. Müller CS, Körner R, Takacs FZ, Solomayer EF, Vogt T, Pfoehler C, Metastatic breast carcinoma mimicking a sebaceous gland neoplasm: a case reportJ Med Case Rep 2011; 5: 428-
15. Hisaoka M, Takamatsu Y, Hirano Y, Maeda H, Hamada T, Sebaceous carcinoma of the breast: case report and review of the literatureVirchows Arch 2006; 449: 484-8.
16. Bhandari V, Gunasekeran G, Naik D, Yadav AK, Infiltrating ductal carcinoma of the breast presenting as breast abscessNat J Med Res 2013; 3: 422-3.
Notes
Source of Support: Nil
Conflict of Interest: None declared.
Comments are closed.