|
Get Citation
|
|
|
Alexandrova AK, Smolyannikova VA, Filatova VA, Alexandrova OK. Protein p16 role in seborrheic keratosis. Our Dermatol Online. 2016;7(4):377-380. |
|
|
Download citation file:
|
Protein p16 role in seborrheic keratosis
Alexandra K. Alexandrova1, Vera A. Smolyannikova1, Varvara A. Filatova1, Olga K. Alexandrova2,
1Department of Pathological Anatomy, Russian Ministry of Health I.M. Sechenov First Moscow State Medical University, Moscow, Russia, 2Department of Infectious Diseases and Epidemiology, Kuban State Medical University, 350063, Sedin street 4, Krasnodar, Russia
ABSTRACT
Introduction: Seborrheic keratosis (SK) is a disease of unknown etiology and pathogenesis. Keratomas grow slowly for years. Their number grows significantly with age in some patients, resulting in cosmetic defect, while separate elements remain in others not disturbing them subjectively. Details of the cell cycle destruction by the SK are not revealed despite a number of studies. It is controversial whether tumor suppressor protein p16 influences growth and development of the tumor elements.
Materials and Methods: An immunohistochemistry test with monoclonal antibodies to p16 was accomplished, 20 SK served as a material for the test, which were obtained from patients with multiple SK – 10 people, and single SK (not more than 10 elements on the skin) – 10 people. Clinical examination of patients was being conducted, using data from the anamnesis of concomitant somatic pathology.
Results: Intense cytoplasmic and nuclear staining of tumor cells was revealed by individuals with multiple SK in 70% by immunohistochemical test with monoclonal antibodies to p16, 30% of the staining was moderate, diffuse. A positive reaction with antibody to p16 was diffuse, weak by patients with single SK in 80% of the cases, stain of the single cells of the basal layer nucleus was recorded, in 20% the colour of the cells cytoplasm was intense, but as separate focuses. The presence of insulin resistance was revealed from anamnesis by all patients with multiple SK. Insulin resistance by patients with single SK was detected in 2 cases.
Conclusion: The connection was found between the intensity of the p16 expression and the prevalence of SK. Given the presence of insulin resistance in the anamnesis of patients with multiple SK, an assumption was made about an indirect effect of the p16 expression on hyperinsulinemia. The presence of focal intense reactions with antibodies to p16 by patients with single SK can serve as a predictor of eruptions dissemination in future.
Key words: Seborrheic keratosis; Immunohistochemistry; Tumor suppressor protein p16; Insulin
INTRODUCTION
Seborrheic keratosis (SK) is a benign epithelial tumor, occurring mostly in patients after 35-40 years old, with unclear etiology. The role of such factors as increased sun exposure, carcinogens, human papilloma virus, genetic predisposition has not been proven in the development of SK [1,2]. Immunohistochemictry test (IHC test) conducted to examine cell cycle regulators (cyclin-dependent kinases and their inhibitors, growth factors) disclosed violations of the cell cycle by SK. The details of violations however remained unclear [3,4].
A theory of keratinocytes “aging” finds more supporters when seborrheic keratoses. The aging prevents unlimited and uncontrolled growth of damaged cells, thereby preventing degeneration, but on the other hand, increasing the cells resistance to apoptosis [5]. The main genes responsible for the process of “aging” are genes which produce proteins p16 and p53. In human body the p16 is encoded by the gene CDKN2A, located on chromosome 9 (9p21.3) [5–7]. The protein p16 was discovered by researchers in 1993 [8]. This gene is involved in the development of both sporadic and familial melanoma, glioma, when lung cancer, T-cell leukemia. In addition, p16 is currently studying as a prognostic biomarker for patients with squamous cell carcinoma of the oropharynx, cervical cancer [9,10].
The ρ16/INK4 gene is a tumor suppressor gene belonging to the group of “cell cycle guardians”. At the molecular level, the effect of the p16 protein is based on the inhibition of the cell cycle regulators [11]. During the G1 phase, the activity of cyclin-dependent kinases in a growing cell (Cdk) is blocked until the cell starts the next cell cycle [12].
Inhibitors of cyclin-dependent kinases are divided into two main families: Cip/Kip and INK4. The INK4 family inhibitors block the Cdk4 and Cdk6 cyclin-dependent kinases which control the G1 cell cycle phase. The Cip/Kip family inhibitors block the cyclin-dependent kinase in conjunction with cyclin [7]. The p16 protein is a cyclin-dependent kinase inhibitor participating in the Rb/cyclinD/cdk4/p16INK4a biochemical pathway [13]. p16 is bound to kinases such as CDK4 and CDK6, and this complex upsets the interaction between kinases and cyclin D [14]. Inhibition of the functions of the cyclin-dependent kinases results in the hypophosphorylation of the pRB protein which reduces the expression of E2F-dependent genes and blocks cell transition from phase G1 to phase S ensuring cell division and proliferation control [9,15]. Many sources confirm that disturbances in the G1 phase and G1/S control point result in uncontrolled tumor growth [12]. It is shown by experiments that a reduced expression of p16 results in pRB hyperphosphorylation [14]. In malignant tumors, cells overcome an increased expression of the p16 gene through its inactivation, by using hypermethylation or deletion. Thus, the effect of that gene and its product, the p16 protein, combines differently directed processes: oncogenesis and aging, fixing them at the opposite ends. At one end, hypermethylation, mutations, and deletions of p16 result in gene control disruption and cancer development through disrupted cell cycle control. Conversely, p16 activation through DNA damage results in its accumulation in tissues, suppressed proliferative activity, and cellular senescence [15].
MATERIALS AND METHODS
Twenty tumors were used as study materials which were surgically removed from 10 patients with multiple SKs (more than 10 elements) and 10 patients with single SKs. The SK diagnosis was made on the basis of the results of clinicopathologic studies. The age of the sick people varied from 46 to 75 years, with women prevailing (70%). The most typical elements of seborrheic keratosis on the body skin were selected for studies, a maximum of 2 cm in diameter, flat, moderately pigmented, with a hyperkeratotic surface. The operational material was fixed in the 10% neutral buffered formalin solution, with subsequent preparation of paraffin blocks. The sections were stained with haematoxylin and eosin. Primary monoclonal antibodies of p16 were used for IHC tests, at a dilution of 1:200 (Novocastra Laboratories Ltd.). The IHC reaction results were evaluated by color intensity (pronounced, moderate, weak) and by prevalence of cytoplasmic or nuclear staining.
RESULTS
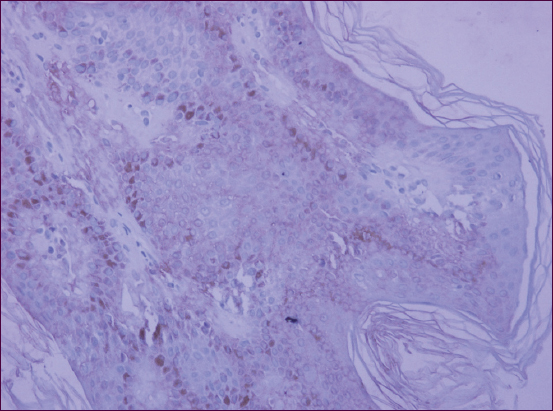
High-grade diffused cytoplasmic response of antibodies to p16 was reported for 7 patients from the group having multiple SKs (70%) (Fig. 1). In 3 cases (30%), moderate-intensity cytoplasmic staining of the entire tumor tissue was identified. Also, nuclear expression of p16 was present in all cases, essentially for basal layer cells.
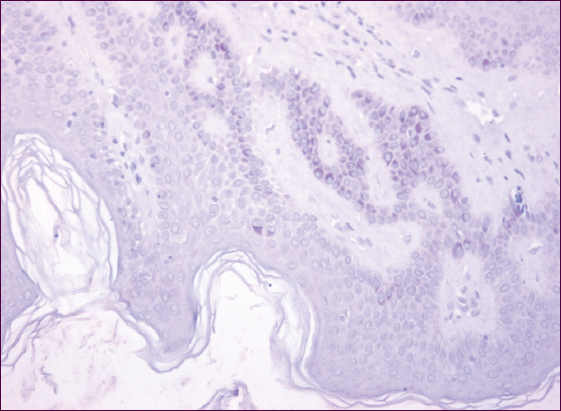
For the group having single SKs, weak diffused cytoplasmic staining of tumor cells was reported for 8 persons (80%), with a positive response in individual nuclei (Fig. 2). Intensive focal staining of the cell cytoplasm was noted in 20% of cases. It was established from the patients’ medical histories that all patients having multiple SKs were insulin resistant, with 5 persons followed up for pancreatic diabetes. There were 2 insulin resistance cases registered for the persons having single SKs.
DISCUSSION
Different foreign authors treat differently the p16 expression difference and its role in the cell cycle. It is considered that p16 expression increases substantially with age which may be a marker of cellular senescence at the molecular level [16,17]. We did not reveal any clear-cut relation between age and a more pronounced p16 expression in our study; both patient groups were equal in terms of age structure which gives no reason to believe that an increased p16 is primarily explained by age. Nor was the opinion that p16 is a possible malignant transformation marker for SK elements confirmed. Thus, in studying 17 cases of seborrheic keratosis with signs of Bowenoid transformation, Wu YH et al. discovered an increased expression of p16 and p21 in cells which is also typical for the Bowen disease and Bowenoid papulosis. It was suggested that they study p16 for patients having seborrheic keratosis to identify possible malignant transformation of elements [5]. In our opinion, for that suggestion to be made, there should be other undoubted morphological signs of a malignancy in place. Other publications, in particular, Nakamura S. et al., revealed a pronounced expression of the p16 protein in all tumor cells for skin samples from the SK affected area and associated it with a disrupted cell cycle, blocking of the cell S phase entry, and their premature aging, i.e. with apoptosis inhibition, rather than with possible malignant transformation [3]. However, in the study by Brueks et al. of 10 acantholytic nevus cases of the acanthotic and irritated types, the p16 protein expression level was medium and, in the author’s opinion, has no significant role in SK pathogenesis [4].
The results of our study showed the following distinctive feature: a significant expression of p16, both cytoplasmic and nuclear, was reported for 70% of the persons having multiple SKs who were also diagnosed to be insulin resistant. A question arises concerning a possible indirect effect of hyperinsulinemia on p16 expression. Literature contains data indicating an increased level of p16 in the pancreatic gland during aging which inhibits proliferation of beta cells and reduces their ability to respond to damage. This is one of the possible development mechanisms for “old age” diseases, such as pancreatic diabetes, CAD, apoplectic attack, etc. In our opinion, hyperinsulinemia causes an increased proliferative activity of cells entailing an increased expression of the p16 suppressor protein and kinase inhibitor.
CONCLUSIONS
Based on p16 expression, one can assess the occurrence of rashes for SK patients, given its substantial amount identified for the persons having multiple elements. We consider the presence of an intensive focal reaction with antibodies to p16 for 2 single SK patients as a marker of further disease progression and the number of tumors eventually increasing. The interrelation between an increased expression of p16 and insulin resistance requires a further study to better understand the disease pathogenesis and expand treatment options.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
REFERENCES
1. Hafner C, Vogt T, Seborrheic keratosisJ Dtsch Dermatol Ges 2008; 6: 664-77.
2. Hafner C, Vogt T, Landthaler M, Müsebeck J, Somatic FGFR3 and PIK3CA mutations are present in familial seborrhoeic keratosesBr J Dermatol 2008; 159: 214-7.
3. Nakamura S, Nishioka K, Enhanced expression of p16 in seborrhoeic keratosis;a lesion of accumulated senescent epidermal cells in G1 arrestBr J Dermatol 2003; 149: 560-5.
4. Bruecks AK, Kalia S, Trotter MJ, Overexpression of p27KIP1 in seborrheic keratosisJ Cutan Med Surg 2007; 11: 174-8.
5. Wolff K, Goldsmith LA, Fitzpatricks Dermatology in General Medicine 2012; Seventh edition. McGraw-Hill Medical;
6. Wu YH, Hsiao PF, Chen CK, Seborrheic Keratosis With Bowenoid Transformation: The Immunohistochemical Features and Its Association With Human Papillomavirus InfectionAm J Dermatopathol 2015; 17: 24-5.
7. Harvey LM, Molecular Cell Biology 2008; 6th. New York City: W.H. Freeman and Company;
8. Liggett WH, Sidransky D, Role of the p16 tumor suppressor gene in cancerJ Clin Oncol 1998; 16: 1197-206.
9. Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA, Role of the INK4a locus in tumor suppression and cell mortalityCell 1996; 85: 27-
10. Cioffi-Lavina M, Chapman-Fredricks J, Gomez-Fernandez C, Ganjei-Azar P, Manoharan M, Jorda M, P16 expression in squamous cell carcinomas of cervix and bladderAppl Immunohistochem Mol Morphol 2010; 18: 344-7.
11. Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA, Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancersNature 1994; 368: 753-55.
12. Morgan D, The Cell Cycle: Principals of Control 2007; London: New Science Press LTD;
13. Dyson N, Baiman A, Oncogenes and cell proliferationCurr Opin Genet Dev 1999; 9: 11-4.
14. Rosso AA, Tong L, Lee JO, Jeffrey PD, Pavletich NP, Struktural basis for inhibition of the ciklin-dependent kinase Cdk6 by the tumour suppressor p16/INK4ANature 1998; 395: 237-43.
15. Rayess H, Wang MB, Srivatsan ES, Cellular senescence and tumor suppressor gene 16Int J Cancer 2012; 130: 171-4.
16. Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human agingAging Cell 2009; 8: 439-48.
17. Roussel MF, The INK4 family of cell cycle inhibitors in cancerOncogene 1999; 18: 5311-12.
Notes
Source of Support: Nil
Conflict of Interest: None declared.
Comments are closed.