Giant primary melanoma of the skin arising on the left foot
Vladimír Bartoš1, Zuzana Štofová2
1Department of Pathology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, Slovakia, 2Department of Clinical Oncology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, Slovakia
ABSTRACT
Melanoma of the skin usually does not reach a greater dimension. The authors describe unique case of the patient with a huge (giant) primary cutaneous melanoma. 56-year-old man was diagnosed to have melanoma arising on the left foot. Partial surgical amputation with a complete tumor removal was done. Melanoma measured 8 × 4 cm and exhibited massive ulceration, Breslow’s thickness 15 mm, Clark’s level V, high mitotic and proliferative activity. A patient was classified as stage III B. After surgery, he was relegated to the oncological dispensary health care and took part additional clinical investigations. Finally, PET/CT scan revealed pulmonary and regional lymph node metastases. Therefore, paliative chemotherapy was started. Huge skin melanomas like this are very rarely diagnosed in clinical practice and they are almost always the result of long-term neglect of growing lesion. Currently, it is not answered, whether certain specific factors predispose some melanomas to grow such enormously.
Key words: Malignant melanoma; Biological behaviour; Foot
INTRODUCTION
Melanoma of the skin is one of the most aggressive and prognostically the most unfavorable malignancies in humans. Currently, it is extensively studied oncological entity, mostly due to its dramatically increasing incidence all over the world [1,2]. Cutaneous melanoma usually does not reach a greater dimension. In recent analysis, Seidenari et al. [3] found a mean (microscopically verified) horizontal diameter 1.06 cm. In contrast to skin carcinomas, pathological TNM (Tumor, Nodes, Metastasis) classification of cutaneous melanoma [4] does not include horizontal, but vertical dimension (Breslow’s thickness), which is prognostically much more important. Considering an aggressive biological behaviour and high metastatic potential of melanoma there is a small chance, it could grow to the greater size. Moreover, some melanomas may also completely regress, what is usually accompanied by metastases [5]. Therefore, a presence of metastasis of malignant melanoma with unknown primary origo is not unique finding in clinical practice [6,7]. Anyway, medical literature has sporadically documented case reports of huge (so-called giant) melanomas, although their cut-off limit has not been consensually defined. Some papers [8,9] have considered giant melanomas those, diameter of which exceed 10 cm. However, several authors [10–15] have described cases of giant melanomas not reaching this size. Herein, we present a patient with a huge (giant) cutaneous melanoma arising on the foot.
CASE REPORT
A 56-year-old man presented with a huge solid tumor involving the toes and instep of the left foot. He admitted the lesion was long standing and originally started to arise on the skin between the fourth and fifth toe almost 3 years ago. Initially, it was asymptomatic and painless. During the last months, however, tumor has began to grow rapidly, progressively increased in size and was accompanied by extensive ulceration, intermittent bleeding and pain. Therefore, he had to see a medical attention and visit an ambulatory surgeon (March, 2015), who performed a probatory incision. After getting a histologically proven diagnosis, he reffered him to the hospital. Subsequently, a patient was admitted to the Department of Surgery, where a partial amputation of the foot was preferentially planned. Routine preoperative clinical investigations were done. Among them, none revealed an evidence of distant metastases. X-ray imaging of affected leg did not confirm a tumor infiltration of the bone tissue. A chest X-ray showed no obvious pathology in the pulmonary parenchyma. The standard laboratory parameters were (except slightly elevated serum glucose) within normal limits.
A partial surgical amputation and exarticulation of the foot was done, biopsy specimen was immediately fixed in formalin and sent for definitive histopathological examination. A received biopsy sample consisted of a part of the foot with three toes. On macroscopic examination, the toes, interdigital and metatarsal regions were massively infiltrated by gray-brownish tumor mass. The fourth toe was affected most severely, it was twisted and deformated. A tumor measured 8 × 4 cm in greatest dimension, it was irregularly shaped, protuberant and ulcerated. All nails were intact without tumor changes. There were four colored surgical stitches fixed on the basis of the sample to better indicate resection margins.
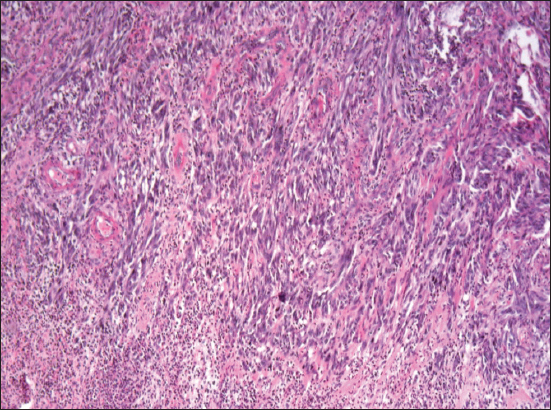
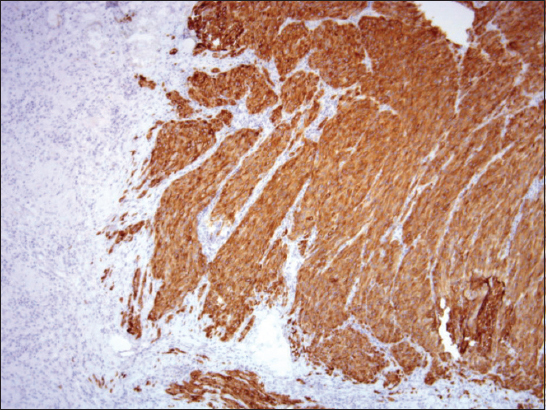
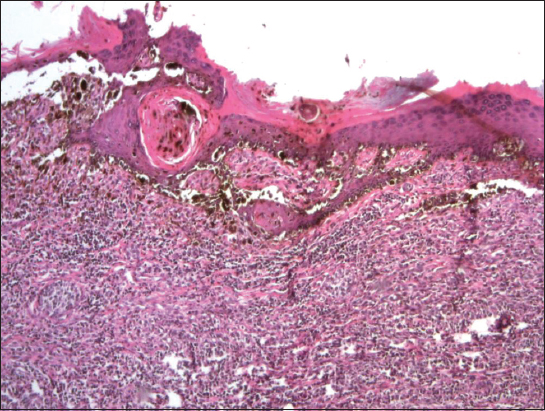
Histopathology revealed a primary malignant melanoma predominantly composed of atypical spindle-shaped cells (Figure 1). It was markedly ulcerated with a purulent detritus on the ulcer basis. Breslow’s thickness was at least 15 mm and Clark’s level V. Immunohistochemically, it was strongly positive for melan A (Figure 2), HMB-45, S-100 protein and negative for polyclonal cytokeratins. Disseminated deposits of melanin pigment were present in the entire sections. Mitotic activity reached 25 mitoses per 1 mm2. Proliferative activity (Ki-67 index) was also high (cca 40%). Neither lymphovascular nor perineural tumor invasion was found in excised sections. In one region, small tumor satellite occurred 8 mm from the main tumor mass. There was scattered intratumorous lymphocyte infiltration (TIL +). Because of advanced stage of the lesion, it was not possible to clearly determine an original histological type of melanoma. However, in one histological section, a small focus of residual atypical lentiginous melanocyte proliferation was detected (Figure 3), which might suggest (even in the contex with anatomical site) an acral lentiginous type. According to the UICC (Union for International Cancer Control) TNM staging system [4], the patient was classified as stage III B (pT4b,N2c,MX). All resection margins marked with surgical stitches were free of tumor and a minimum of 15-mm clearance was achieved. Surrounding skin tissue did not exhibit a solar damage.
Shortly after surgery, CT (computed tomography) scan of thoracic cavity and mediastinum was realized. It confirmed multiple intraparenchymatous nodular lesions in both lungs, some of which unsharply demarcated. The largest one measured 13 × 11 mm and grew out the pleural surface. Thus, melanoma metastases or mycotic infection were considered in differential diagnosis. Furthermore, enlarged lymph node (15 × 11 mm) in the right pulmonary hilus was visible. CT imaging of the abdomen and pelvic cavity did not reveal obvious pathological changes in the visceral organs, either intraabdominal lymphadenopathy. Similarly, both groins were without evident enlargement of the lymph nodes. A postoperative course was uneventful, the patient left the hospital and consequently, he was relegated to the oncologic dispensary health care. An oncologist recommended further examinations to elucidate the origin of pathologic nodules in the lungs. Bronchoscopy did not confirm persuasive pathological changes within the bronchial tree. An aspiration lavage was done and sent for cytological analysis, but no tumor cells nor mycotic microorganisms were found in the fluid. Therefore, the patient underwent PET/CT (positron emission tomography – computed tomography) examination, that showed an increased glucose metabolism (FDG, 2-fluoro-2-deoxy-D-glucose) within the nodules in both lungs, as well as in the left groin. Based on clinically very suspcicious evidence of metastases, a paliative chemotherapy was started. At the time this report was written, our patient had underwent monochemotherapy with dacarbazine. Additionally, there was not detected BRAF gene mutation in tumor tissue.
Prior to the study, patient gave written consent to the examination and biopsy after having been informed about the procedure.
DISCUSSION
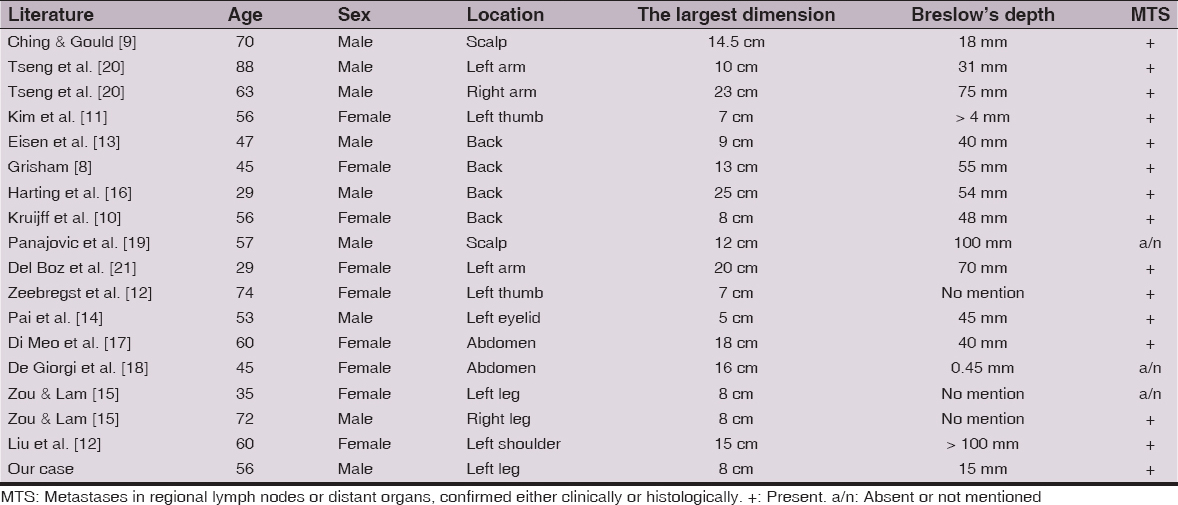
Huge skin melanomas like this are very rarely diagnosed in routine clinical practice, mainly because the patients tend to come for treatment early before the tumor enlarges. Therefore, they are almost always the result of long-term neglect of growing lesion. An overview of the cases that have been published until now, including our present case report are summarized in Table 1. As may be seen, they usually occur in the middle or elderly age with approximatelly equal sex distribution. However, sometimes have been observed in young people up to 35 years of age. In the most documented cases they were localized on the trunk [8,10,13,16–18], but involved also other sites, such as scalp [9,19], arm [20,21], shoulder [22], thumb [11,12], and eyelid [14]. To the best of our knowledge, there has been published only one paper describing giant cutaneous melanoma involving the foot. Zou and Lam [15] briefly introduced two patients with nodular malignant melanoma located on the sole, both reaching the largest diameter of 8 cm.
According to the literature data [17], a majority of giant melanomas of the skin grew up primarily (de novo) without association with precursor melanocytic lesion. Occasionally, there have been described the cases arising from congenital [16] or acquired pigmented melanocytic nevus [14,21]. In our case, it was likely to develop de novo, because a patient did not state a previous pigmented skin lesion on the incriminated region. In advanced melanomas, it is very difficult (and usually impossible) to determine an original histological type. Therefore, several case reports of giant melanomas have not precisely classified an histological type. We suppose that our patient could originally have acral lentiginous melanoma, which is the most typical for this anatomical site. In addition, microscopic features of atypical junctional lentiginous melanocytic proliferation were detected at the periphery of the tumor mass. Anyway, in such advanced stages, an exact histological typing does not play a prognostical significance. It is not surprising, a majority of reported giant melanomas were accompanied by metastases in regional lymph nodes or distant organs, whether detected within primary tumor diagnosis, or during further investigations. Except two case reports [18,19], all others corresponded to the clinical stage III or IV and the patients were usually subsequently treated with paliative modalities (i.e. chemotherapy or a-interferon immunotherapy). However, in such cases, cure effect, as well as disease outcome and survival are hardly predictable.
In conclusion, it is not clear, whether melanomas, which are capable to progress in such large dimension exhibit different biological phenotype from that of “usual” melanoma of equivalent thickness. Further, we have insufficient knowledge, whether certain specific factors predispose some melanomas to grow such enormously. Some melanoma patients with much more smaller primary lesions behave poorly with early distant metastasis, while others develop very extensive primary tumors without or with only late metastases. In addition, some melanomas may fully regress and this phenomenon is usually accompanied by metastatic spread. More light remains to be shed on the pathobiology, malignant progression and pathways of metastasis in this neoplasia, as they are still only partially understood.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Kuchár Pavol (surgeon) for assistance concerning the clinical information and Dr. Doboszová Jana (pathologist) for technical assistance.
CONSENT
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Godar DE, Worldwide increasing incidences of cutaneous malignant melanomaJ Skin Cancer 2011; 2011: 858425.
2. Bartoš V, Kullová M, Age-related differences in the incidence and clinicopathological findings of malignant melanoma of the skinOur Dermatol Online 2015; 6: 140-4.
3. Seidenari S, Fabiano A, Al Jalbout S, Bassoli S, Borsari S, Magnoni C, Relationship between histological and computer based assessment of melanoma diameter and thickness in head & neck vs. trunk melanomaHead Neck Oncology 2013; 5: 32.
4. Sobin LH, Gospodarowicz MK, Wittekind Ch, TNM Classification of Malignant Tumours 2009; 7th Edition. Wiley-Blackwell; 336 pages-ISBN: 978-1-4443-3241-4
5. High WA, Stewart D, Wilbers CR, Cockerell CJ, Hoang MP, Fitzpatrick JE, Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteriaJ Am Acad Dermatol 2005; 53: 89-100.
6. Brzeziński P, Abdulaziz A, Andruszkiewicz J, Chiriac A, Malignant melanoma of unknown primary site in patient with pustulosis plantarisOncoReview 2014; 4: A69-71.
7. Savoia P, Fava P, Osella-Abate S, Nardò T, Comessatti A, Quaglino P, Melanoma of unknown primary site: a 33-year experience at the Turin Melanoma CentreMelanoma Res 2010; 20: 227-32.
8. Grisham AD, Giant melanoma: novel problem, same approachSouth Med J 2010; 103: 1161-2.
9. Ching JA, Gould L, Giant scalp melanoma: a case report and review of the literatureEplasty 2012; 12: e51.
10. Kruijff S, Vink R, Klaase J, Salvage surgery for a giant melanoma on the backRare Tumors 2011; 3: e28.
11. Kim JH, Jeong SY, Shin JB, Ro KW, Seo SH, Son SW, Giant acral melanoma on the left thumb of a korean patientAnn Dermatol 2009; 21: 171-3.
12. Zeebregts CJ, Schraffordt Koops H, Giant melanoma of the left thumbEur J Surg Oncol 2000; 26: 189-90.
13. Eisen DB, Lack EE, Boisvert M, Nigra TP, Giant tumor of the backArch Dermatol 2002; 138: 1245-50.
14. Pai RR, Kini H, Kamath SG, Kumar S, Giant hanging melanoma of the eyelid skinIndian J Ophthalmol 2008; 56: 239-40.
15. Zou Y, Lam A, Giant primary plantar melanomaJ Eur Acad Dermatol Venereol 2009; 23: 361.
16. Harting M, Tarrant W, Kovitz CA, Rosen T, Harting MT, Souchon E, Massive nodular melanoma: a case reportDermatol Online J 2007; 13: 7.
17. Di Meo N, Stingo G, Gatti A, Errichetti E, Bonin S, Albano A, Giant melanoma of the abdomen: case report and revision of the published casesDermatol Online J 2007; 20: pii: 13030/qt4pp2825w
18. De Giorgi V, Massi D, Carli P, Giant melanoma displaying gross features reproducing parameters seen on dermoscopyDermatol Surg 2002; 28: 646-7.
19. Panajotovic L, Dordevic B, Pavlovic MD, A giant primary cutaneous melanoma of the scalp – can it be that big ?J Eur Acad Dermatol Venereol 2007; 21: 1417-8.
20. Tseng WW, Doyle JA, Maguiness S, Horvai AE, Kashani-Sabet M, Leong SP, Giant cutaneous melanomas: evidence for primary tumour induced dormancy in metastatic sites?BMJ Case Rep 2009; 2009: pii: bcr07.2009.2073
21. Del Boz J, Garcia JM, Martinez S, Gomez M, Giant melanoma and depressionAm J Clin Dermatol 2009; 10: 419-20.
22. Liu F, Kong LM, Ng S, Hunter-Smith DJ, Findlay MW, Massive primary melanoma without clinically detectable metastasesANZ J Surg 2015; 85: 688-9.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Comments are closed.