Weekly methotrexate versus daily isotretinoin to treat moderate-to-severe chronic plaque psoriasis: A comparative study
C. Abhinav, Vikram K Mahajan, Karaninder S. Mehta, Pushpinder S. Chauhan, Mrinal Gupta, Ritu Rawat
Department of Dermatology, Venereology and Leprosy, Dr. R. P. Govt. Medical College, Kangra (Tanda)-176001 (Himachal Pradesh), India
ABSTRACT
Introduction: There is paucity of data on efficacy of isotretinoin versus methotrexate in psoriasis patients.
Aims: We compared the efficacy and safety of once a week methotrexate and daily isotretinoin for the treatment of moderate-to-severe chronic plaque psoriasis.
Methods: 43 (M: F 30:13) consenting patients having moderate-to-severe chronic plaque psoriasis were divided on alternate basis in Group-A for treatment with methotrexate (15mg/week) and Group-B for isotretinoin (30mg/d) therapy. The clinical response was assessed by the percentage reduction in the baseline PASI scores during next 12 weeks.
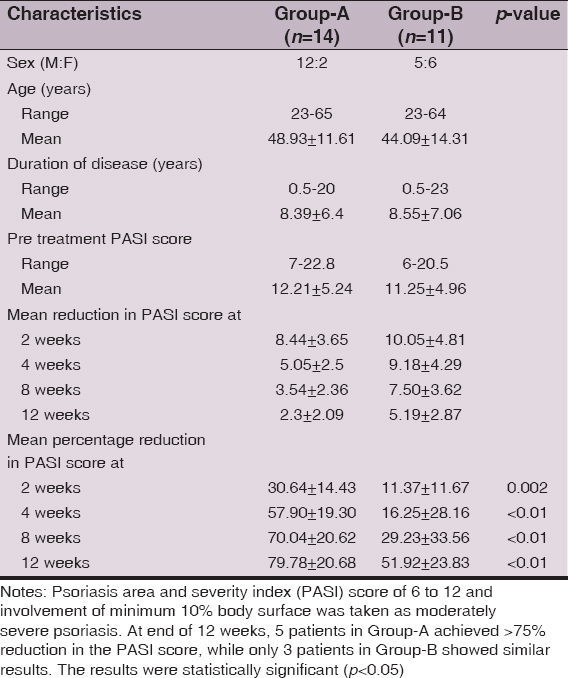
Results: 14 patients in Group-A and 11 patients in Group-B completed the study. The mean percentage reduction in the PASI score was 79.78 ± 20.68 in the methotrexate group and 51.92 ± 23.83 in the isotoin group at the end of 12 weeks. Five patients in Group-A achieved >75% reduction in the PASI score, while only 3 patients in Group-B showed similar results at the end of 12 weeks signifying faster disease clearance with methotrexate. Isotretinoin-related serious adverse effects were fewer and did not warrant treatment discontinuation.
Conclusions: Isotretinoin may be an option for alternative therapy in patients who cannot afford acetretin, are intolerant to methotrexate, have achieved its cumulative dose, or in rotational therapy.
Key words: Isotretinoin; Methotrexate; Psoriasis; Retinoids
INTRODUCTION
Chornic plaque psoriasis is a common, genetically determined, inflammatory and proliferative dermatological disorder with an estimated prevalence of 1.5-3.0% in Europe and 2.2-2.6% in US [1,2]. Immunologically mediated activation of T lymphocytes is central to the inflammation in the dermal microenvironment and epidermal hyper-proliferation occurs secondary to the inflammatory events that follows a Th1 type of immune response [3]. It affects both genders at any age and has chronic and unpredictable clinical course adversely affecting quality of life. However, despite a substantial progress toward elucidation of genetic and pathophysiological pathways involved in psoriasis, a safe affordable and effective/curative therapy remains desirable. Most topical medications (emollients, tars, anthralins, corticosteroids, retinoids (tazarotene), vitamin-D analogues (calcipotriol, calcitriol, tacalcitol, maxacalcitol), topical calcineurin inhibitors (tacrolimus, pimacrolimus), narrowband UVB phototherapy) or systemic therapies (methotrexate, retinoids, cyclosporine A, PUVA (psoralens+UVA), hydroxyurea, biologics), used alone or in combination with topical therapies, control the severity and extent of psoriasis. These therapies are associated with potentially severe toxicities, require extensive monitoring and are expensive [1,4]. The biologics (alefacept, infliximab, adalimumab, etanercept, efalizumab) offer better safety profile but cost and availability remain prohibitory. Despite concerns for hepatotoxicity, methotrexate (7.5mg to 30mg/week) remains standard systemic therapy for moderate to severe psoriasis unresponsive to topical therapy, in psoriatic arthritis and nails psoriasis due to affordability, high efficacy and fast results [1,5]. There has been a continuing search for a less toxic, but equally effective replacement for methotrexate once its cumulative dose (1.0-1.5 gm) is achieved [6]. A rapid and impressive response may be seen with retinoids in pustular psoriasis and erythrodermic psoriasis. Etretinate, the first retinoid, was withdrawn because of potential risk of teratogenicity due to its long half-life. Acitretin, the active metabolite of etretinate, has a shorter half-life and can be taken for long when not planning pregnancy for 3 years after its discontinuation [7]. However, high cost of acitretin precludes its routine use in most instances. Comparatively, isotretinoin is cheaper and has shorter half-life and contraception is required during therapy and 1 month thereafter [8,9]. Although considered more effective in pustular psoriasis (8-10), its efficacy in chronic plaque psoriasis remains under studied. Moy et al [10] found acitretin (0.75 mg/kg/d for 8 weeks) effective in 18/19 of chronic plaque psoriasis and also achieved complete to moderate response with isotretinoin (1.5 mg/kg/d) in their 4/10 patients with chronic plaque psoriasis involving 20-50% body surface area when given for the same period. However, there is overall paucity of data on efficacy of isotretinoin in patients having moderate to severe chronic plaque psoriasis. In this prospective study, we compared the efficacy and safety of once a week methotrexate and daily isotretinoin for the treatment of moderate to severe chronic plaque psoriasis.
MATERIAL AND METHODS
Forty three consenting patients of psoriasis presenting with moderate to severe psoriasis over a period of one year were enrolled after written/informed consent. PsoriasisArea-and-Severity Index (PASI) score of 6-12 or body surface area (BSA) involvement up to 10% was considered moderate psoriasis while PASI score >12 or BSA involvement >10% was considered severe psoriasis [11]. The study was approved by the Institutional Protocol Review Board and Institutional Ethics Committee (Rgn no. ECR/490/Inst/HP/2013). Patients <18 years of age, having BMI >30 or weighing <30 kg, women who were pregnant, lactating or unwilling for an effective regimen of contraception, patients with psoriatic arthritis, any systemic disease (hepatorenal or hematologic impairment, deranged lipid profile acute infection requiring antimicrobial therapy, uncontrolled hypertension or diabetes mellitus, neoplasias, psychiatric illness), or a long history of alcohol intake were excluded. A detailed history and clinical examination were recorded in all eligible patients. Laboratory investigations were performed in all patients, biweekly during the first month and every month during the next two months and included complete blood counts, fasting blood sugar, lipid profile, serum creatinine, serum urea, serum glutamine pyruvate transaminase (SGPT), serum glutamine oxalate transaminase (SGOT), alkaline phosphatase, and serum bilirubin (total and conjugated). Chest X-ray and serum electrolytes were performed at the beginning and at the end of the treatment in each patient.
Forty-three patients were eligible for the study. They were assigned alternately to Group-A for weekly dose of methotrexate (Methotrexate Group) or Group-B for daily dose of isotretinoin (Isotretinoin Group) with option to withdraw any time from the study. Patients already on any antipsoriasis treatment were given a wash off period of 2 and 4 weeks for topical and systemic therapy respectively. Photographic records were kept in all patients before and after the treatment.
Treatment Protocols
Group-A patients received once a week oral methotrexate 15 mg (given in two divided doses at an interval of 12 hours) and Group-B patients received isotretinoin 30 mg/d PO. Patients were evaluated every two weekly for first 4 weeks and once in 4 weeks thereafter for the next 8weeks. Side effects known to be associated with methotrexateor isotretinoin, as well as other side effects that seem relevant to the treatment were evaluated on every visit. The treatment was stopped immediately in the event of intolerable adverse drug reaction. Only emollients and antihistamines in both the groups and folate supplements and anti-emetics for Group-A (methotrexate group) patients were allowed during the study.
Patient Evaluation
Reduction in PASI score was the primary outcome measure. The PASI score, as proposed originally by Fredriksson and Pettersson [12], was determined initially at base line, at 2 weeks during the next month, and at 4 weekly intervals thereafter. The improvement was graded according to reduction in PASI scores as shown in Table 1. PASI scores were compared with respective pretreatment baseline values at end of the study and their percentage reduction was analyzed statistically using paired t-test and independent t-test. A ‘p’ value less than 0.05 calculated at 5% level (95% confidence limits) was considered statistically significant.
Table 1: Grades of improvement by reduction in PASI Score
The study design is registered with Central Trial Registry (CTRI: REF/2013/05/005087).
Ethics
This study was performed on human subjects; thus, all patients were aware of the presence of the study and they were fully informed about the drug and its side-effects.
RESULTS
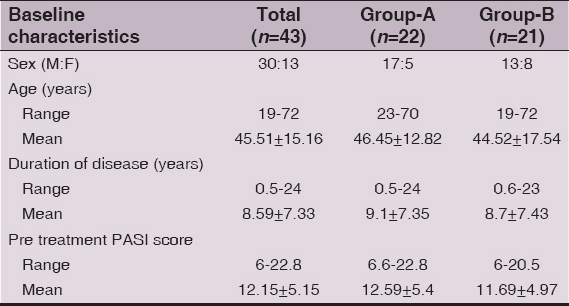
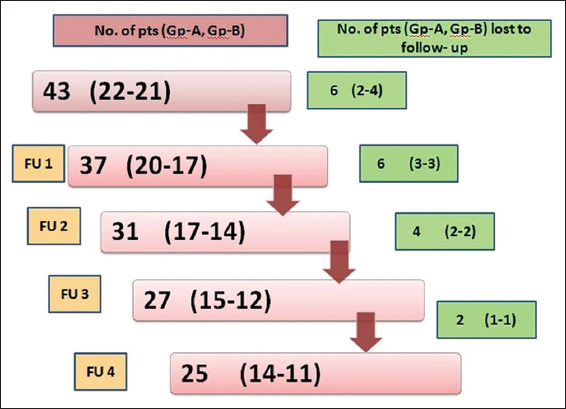
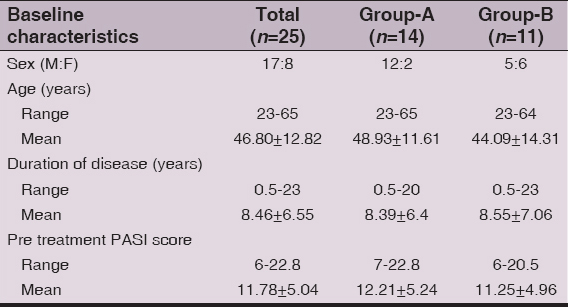
Baseline demographic and disease severity scores of 43 (M: F 30:13) patients are shown in Table 2. The majority, 27(62.8%) patients were aged between 31 and 60 years. The PASI score ranged between 6 and 22.8 (mean 12.15±5.15) and overall duration of psoriasis was 6 months to 24 (mean 8.59±7.33) years. Two patients from Group-B opted for methotrexate treatment due to disease exacerbation or un-satisfaction with treatment response. Other 16 patients dropped out at various stages of the study (Fig. 1). Baseline characteristics of 25 (M: F 17:8) patients, 14 patients in Group-A and 11 patients in Group-B, who completed the study are shown in Table 3.
Table 2: Pretreatment baseline characteristics of all enrolled patients
Table 3: Pretreatment baseline characteristics of the patients who completed the study
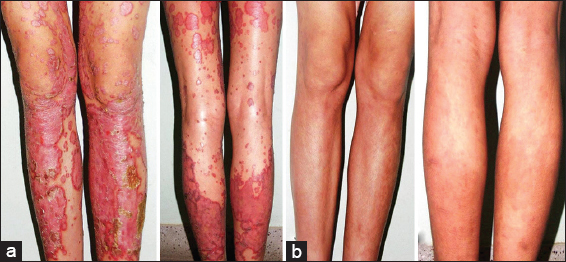
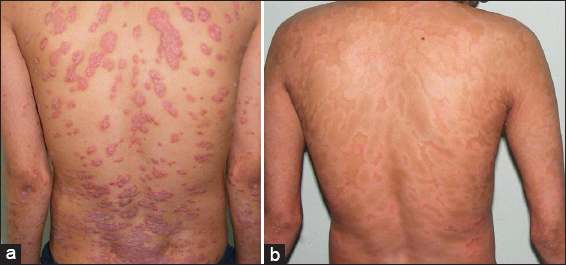
Both methotrexate and isotretinoin were effective in the treatment of psoriasis (Figs 2 and 3). Overall 92% of all patients, 14 patients in Group-A and 9 patients in Group-B, had reached the threshold for a minimal response which means a 25% reduction from baseline in PASI score after 12 weeks of treatment. The mean percentage reduction in the PASI scores of patients from the respective pretreatment levels in both groups was also statistically significant (p <0.05) at each follow up at 2, 4, 8, and 12 weeks and improved progressively as the treatment progressed (Fig. 4, Table 4). Methotrexate was more effective than isotretinoin at all follow up visits as the difference in percentage reduction in PASI score of both groups was statistically significant (p < 0.05) at the end of 2 weeks, 4 weeks, 8 weeks and 12 weeks. At the end of 2 weeks 8 (57.14%) patients in Group-A showed mild improvement while 5 (35.71%) patients were non-responders. However, at the end of four weeks 9 (64.29%) patients showed moderate improvement and 4 (28.57%) patients had mild improvement. Seven (50%) patients continued to improve until end of 8 weeks. At the end of the study 4 (28.57%) patients had marked improvement and 5 (35.71%) patients showed complete remission. Ten (90.9%) patients in Group-B were non-responders initially at 2 weeks but 4 (36.36%) patients among these showed mild improvement at 4 weeks. At the end of 8 weeks mild improvement or no response was observed in 4 (36.36%) patients each. However, at the end of study marked to complete remission, moderate improvement or mild improvement occurred in 3(27.27%) patients each (Fig. 5).
Table 4: Characteristics of the patients who completed the study
Side Effects
Adverse effects from drugs were also noted in both the groups. Eight (57.14%) patients in Group-A experienced methotrexate induced nausea, vomiting, retching and/or anorexia from first month to end of the study period and were managed with antiemetics. Mild abnormality of liver enzymes and thrombocytopenia in one patient each at the end of 8 weeks warranted its discontinuation. However, the patient developing thrombocytopenia had already achieved PASI 75 before discontinuation of methotrexate. Ten (90.9%) patients in Group-B experienced mucocutaneous adverse effects like chelitis, dryness of skin and mouth. One patient complained epigastric discomfort and 4 patients showed altered lipid profile. None of the patient required discontinuation of treatment due to side effects.
DISCUSSION
Psoriasis affects both the genders with some predilection for male but the result vary according to sampling methodology and most studies exclude females [13–15]. Although women may have an early onset, the age of onset varies across reported studies and bimodal distribution has been reported between 16-22 and 57-60 years of age. However, the disease has no phenotypic difference in either sex. Our 43 patients comprised 30 males and 13 females aged 19-72 (mean 45.51 ± 15.16) years and their demographic profile in both the groups were comparable between them and with the reported studies [15–20]. The fewer number of female patients in this study is perhaps for the simple reason of their exclusion during child-bearing age from such studies, small sample size or perhaps that fewer women seek medical treatment at early stage of their disease.
Methotrexate has become gold standard in treatment of psoriasis despite concerns for hepatic toxicity/fibrosis. A plethora of literature exists on its superior efficacy in psoriasis [13–26]. Haustein et al [24] in a 26-year retrospective study had 157 patients with extensive plaque psoriasis or erythrodermic, pustular and arthropathic forms treated with low-dose methotrexate (15-20 mg/week) for long-term periods. The effect of methotrexate was good in 76% patients, moderate in 18% patients and poor in 6% patients and only 20% of cases discontinued the therapy due to side effects. Griffiths et al [27] suggested that methotrexate reduces the severity of psoriasis by at least 50% in at least 75% of patients. Kumar et al [25] reviewed 244 psoriatic patients treated with weekly oral methotrexate from 1981 to 2000 and observed more than 75% improvement in 88% patients in 8.5±5.1 weeks. Weinstein et al [13] and Sandhu et al [17] observed more than 75% improvement in PASI score at the end of 2-3 months and 12 weeks’ treatment with methotrexate. Similarly, Heydendael et al [16] observed complete remission (>90% reduction in baseline PASI score) in their 40% patients and partial remission (>75 % reduction in their baseline PASI score) in 60% patients. The mean pretreatment PASI score in our Group-A patients was 12.21±5.24 and at the end of the study 35.71% patients achieved almost complete remission, 64.29% patients achieved PASI 75 and 85.71% patients achieved PASI 50. The mean percentage reduction in PASI score of 79.78±20.68 after 12 weeks of treatment is comparable to other studies [15,17,19]. Although isotretinoin is considered more effective in pustular psoriasis than in plaque psoriasis [9,10,27,28], there are no studies comparing its efficacy with methotrexate in psoriasis. Anecdotal reports on its use in chronic plaque psoriasis suggest its efficacy as well. Moy et al [10] compared isotretinoin with etretinate in chronic plaque psoriasis and observed moderate to complete response in 4 of 10 patients treated with isotretinoin (1.5 mg/kg/day x 8 weeks). However, isotretinoin has shown equal efficacy to other retinoids when combined with PUVA [29]. The mean pretreatment PASI score in our Group-B patients was 11.25±4.96. The mean percentage reduction in PASI score at the end of 12 weeks was 51.92±23.83 and 3 (27.27%) patients achieved marked to complete remission. Isotretinoin appeared less effective in the first 8 weeks and 4 (36.36%) patients had either mild improvement or were non responder in this study. Overall, the mean percentage reduction of PASI score in our study was higher in methotrexate group than in isotretinoin group at each visit that was statistically significant.
Side Effects
Gastro intestinal (5 patients), mucocutaneous (2 patients), altered liver enzymes (1patient) and thrombocytopenia (1patient) are well known side effects of methotrexate and were observed in 8 (57.14%) patients in Group-A. Flytstom et al [18] observed gastrointestinal side effects as most common adverse effect. van Dooren-Grebbe et al [14] reported methotrexate related side effects (abnormal liver function tests in 44%, nausea in 43%) in their 73% patients. Similarly, Heydendael et al [16] reported nausea in 19(44.19%) patients among 29 (67.44%) patients having gastrointestinal adverse effects. Akhyani et al [17] also observed nausea (80%) and altered liver function test (33.3%) in majority of their patients. Arani et al [20] noted flushing in 2 patients and influenza like symptoms in 7 patients on methotrexate and had to stop methotrexate in 4/25 patients due to liver function abnormalities and recurrent angina. Only in one of our patients, who had marked improvement, treatment was stopped due to thrombocytopenia that recovered after stopping methotrexate. Except for cheilitis (10 patients), altered lipid profile (4 patients) and dry skin/mouth (1 patient each), the well reported adverse effects of isotretinoin, no serious adverse drug reactions warranting discontinuation of treatment were observed in Group-B.
The high dropout rate in the present study was perhaps due to long distance travel for follow up as has been reported often by these patients. Moreover, psoriasis being a chronic disease adherence to treatment or satisfaction level of these patients remains low despite repeated counseling for continuation of therapy and regular follow up. Other reasons for high dropout rate in Group-B were perhaps cost, low efficacy and slow onset of action of isotretinoin as compared to methotrexate.
CONCLUSIONS
Isotretinoin has been used to treat pustular psoriasis primarily and appears to be effective in at least some patients with moderate-to-severe plaque psoriasis. It has fewer serious adverse effects than methotrexate. However, methotrexate is more effective and leads to a faster clearance of the disease as compared to isotretinoin. Isotretinoin may perhaps be one of the options for alternative therapy in patients with mild disease, or who are intolerant to methotrexate, have achieved its cumulative dose, or in rotational therapy when other options are unaffordable, unavailable, or not tolerated. However, better-planned trials are needed to draw any definite conclusion, find full potential of this mode of treatment and devise a standard treatment protocol.
Limitations
It was an un-blinded study and many patients of Group-B had previous history of methotrexate intake. The dose of isotretinoin (30mg/d equivalent to 0.75mg/kg/d for patient averagely weighing 40kg) in this study was at lower end of 0.75-1.5 mg/kg/d used by Moy et al [10]. Possible spontaneous remissions without treatment, small number of patients and high dropout rate of patients at various stages of the study are some of the other limitations to make any recommendation.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
REFERENCES
1. Griffiths CEM, Barker JNWN, Burns T, Breathnach S, Cox N, Griffiths C, PsoriasisRook’s Textbook of Dermatology 2010; 8th ed. Oxford: Blackwell Publishing; 20.1-20.60.
2. Gudjonsson JE, Elder JT, Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, PsoriasisFitzpatrick’s Dermatology in General Medicine 2008; 7th ed. New York: Mc Graw Hill; 169-94.
3. Joshi R, Immunopathogenesis of psoriasisIndian J Dermatol Venereol Leprol 2004; 70: 10-2.
4. Aaronson DS, Lebwohl M, Review of therapy of psoriasis: the prebiologic armamentariumDermatol Clin 2004; 22: 379-88.
5. Roenigk HH, Auerbach R, Malibach H, Weinstein G, Lebwohl M, Methotrexate in psoriasis: consensus conferenceJ Am Acad Dermatol 1998; 38: 478-85.
6. Kalb RE, Strober B, Weinstein G, Lebwohl M, Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus ConferenceJ Am Acad Dermatol 2009; 60: 824-37.
7. Arechalde A, Saurat J-H, Management of psoriasis: the position of retinoid drugsBiodrugs 2000; 13: 327-33.
8. Marhold I, Duschet P, Schwarz T, Gschnait F, Successful use of isotretinoin in Zumbusch generalized pustular psoriasis following recovered etretinate-induced hepatitisHautarz 1991; 42: 580-3.
9. Sofen HL, Moy RL, Lowe NJ, Treatment of generalised pustular psoriasis with isotretinoinLancet 1984; 1: 40.
10. Moy RL, Kingston TP, Lowe NJ, Isotretinoin vs etretinate therapy in generalized pustular and chronic psoriasisArch Dermatol 1985; 121: 1297-301.
11. Feldman SR, A quantitative definition of severe psoriasis for use in clinical trialsJ Dermatolog Treat 2004; 15: 27-9.
12. Fredriksson T, Pettersson U, Severe psoriasis-oral therapy with a new retinoidDermatologica 1978; 157: 238-44.
13. Weinstein GD, Frost P, Methotrexate for psoriasis: a new therapeutic scheduleArch Dermatol 1971; 103: 33-8.
14. van Doore-Greebe RJ, Kuijpers ALA, Mulder J, Boo TD, van de Kerkhof PCM, Methotrexate revisited effects of long-term treatment in psoriasisBr J Dermatol 1994; 130: 204-10.
15. Ranjan N, Sharma NL, Shanker V, Mahajan VK, Tegta GR, Methotrexate versus hydroxycarbamide (hydroxyurea) as a weekly dose to treat moderate-to-severe chronic plaque psoriasis: A comparative studyJ Dermatolog Treat 2007; 18: 295-300.
16. Heydendael VMR, Spuls PI, Opmeer BC, de Borgie CAJM, Reitsma JB, Goldschmidt WFM, Methotrexate versus cyclosporine in moderate to severe chronic plaque psoriasisN Eng J Med 2003; 349: 658-65.
17. Sandhu K, Kaur I, Kumar B, Saraswat A, Efficacy and safety of cyclosporine versus methotrexate in severe psoriasis: a study from north IndiaIndian J Dermatol 2003; 30: 458-63.
18. Flystorm I, Stenberg B, Svensson A, Methotrexate vs cyclosporin in psoriasis: effectiveness, quality of life and safety. A randomised controlled trialBr J Dermatol 2008; 158: 116-21.
19. Akhyani M, Chams-Davatchi C, Hemami MR, Fateh S, Efficacy and safety of mycophenolate mofetil vs. methotrexate for the treatment of chronic plaque psoriasisJ Eur Acad Dermatol Venereol 2010; 24: 1447-51.
20. Arani SF, Neumann H, Hop WCJ, Thio HB, Fumarates vs methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trialBr J Dermatol 2011; 164: 855-61.
21. Bishnoi P, Kumari R, Thappa DM, Monitoring methotrexate hepatotoxicity in psoriasisIndian J Dermatol Venereol Leprol 2011; 77: 545-8.
22. Boffa MJ, Methotrexate for psoriasis: current European practice. A postal surveyJ Eur Acad Dermatol Venerol 2005; 19: 196.
23. van de Kerkhof PCM, Mali JWH, Methotrexate maintenance following Ingram therapy in ‘difficult’ psoriasisBr J Dermatol 1982; 106: 623-7.
24. Haustein UF, Rytter M, Methotrexate in psoriasis: 26 years experience with low dose long-term treatmentJ Eur Acad Dermatol Venerol 2000; 14: 382-8.
25. Kumar B, Saraswat A, Kaur I, Short-term methotrexate therapy in psoriasis: a study of 197 patientsInt J Dermatol 2002; 41: 444-8.
26. Naldi L, Griffiths CEM, Traditional therapies in the management of moderate to severe psoriasis: an assessment of benefits and riskBr J Dermatol 2005; 152: 597-615.
27. Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC, A systematic review of treatments for severe psoriasisHealth Technol Assess 2000; 4: 1-133.
28. Fry L, PsoriasisBr J Dermatol 1988; 119: 445-61.
29. Halverstam CP, Lebwohl M, Non standard and off-label therapies for psoriasisClinic Dermatol 2008; 26: 546-53.
Notes
Source of Support: Nil
Conflict of Interest: None declared.



Comments are closed.