A case of facial lentiginous lichen planus pigmentosus associated with Hashimoto’s thyroiditis and diabetes mellitus
Fadime Kilinc1, Ayse Akbas1, Sertac Sener1, Sibel Orhun Yavuz2, Ayse Akkus3, Akin Aktas3
1Dermatology Clinic, Ataturk Training and Research Hospital, Ankara, Turkey, 2Pathology Clinic, Ataturk Training and Research Hospital, Ankara, Turkey, 3Department of Dermatology, Yildirim Beyazit University Medical Faculty, Ankara, Turkey
ABSTRACT
Lichen planus pigmentosus (LPP) is an autoimmune, chronic and rare variant of lichen planus of unknown etiology that progresses with pigmentation. The condition is rarely observed concurrently with autoimmune diseases. In this case report, a diabetic male patient with speckled lentiginous lesions on the face, also diagnosed with concurrent autoimmune thyroiditis is presented due to the rarity of the condition and the morphological character of the lesions.
Key words: Autoimmune thyroiditis; Hyperpigmentation; Lentiginous; Lichen
INTRODUCTION
Lichen planus pigmentosus (LPP) is an autoimmune, chronic and rare variant of lichen planus of unknown etiology that progresses with pigmentation [1,2]. The lesions usually occur on the skin sections exposed to the sun or on the flexural regions [3]. The pigmentation is usually diffuse, or may be less commonly reticular, speckled, linear or perifollicular [4]. The condition is rarely observed concurrently with autoimmune diseases. In this case report, a diabetic male patient with speckled lentiginous lesions on the face, also diagnosed with concurrent autoimmune thyroiditis is presented due to the rarity of the condition and the morphological character of the lesions.
CASE REPORT
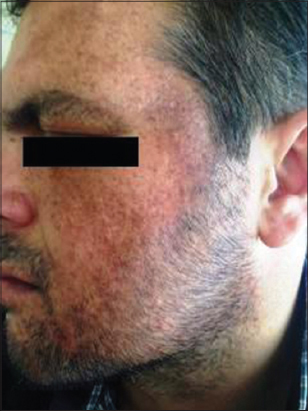
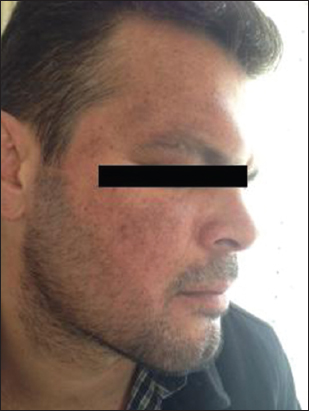
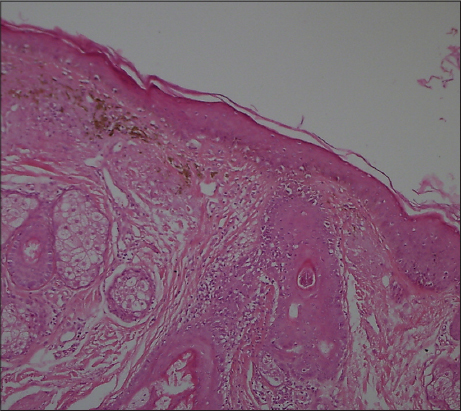
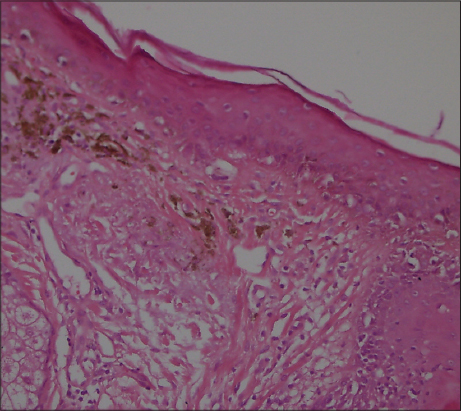
A 41-year old male patient who has been diabetic for the last six years presented to our outpatient clinic approximately four months ago with facial spots that had suddenly appeared on his face. He had apparent multiple, millimetric, lentigo-like brown pigmented macular lesions on his forehead, temples and cheeks (Figs 1 and 2). His oral mucosa, genital area, scalp and nails were normal. He had no pruritus. He had no history of any cosmetic use. He was on metformin treatment for his diabetes, which is known for its photosensitivity-inducing properties. Based on the pre-diagnoses of pigmented lichen planus, Riehl’s melanosis, melasma, and drug-induced hyperpigmentation, a punch biopsy was performed on the pigmented lesion on his face. During the histopathological assessment, the epidermis was observed to be thin with vacuolar degeneration in the basal layer with rare dyskeratotic cells and lymphocytic inflammation in the perifollicular region. Melanophages that have phagocyted melanin have been detected in the papillary dermis (Figs 3 and 4). Based on these findings, the patient was clinically and histopathologically diagnosed with lichen planus pigmentosus. The performed tests also revealed autoimmune thyroiditis (Hashimoto’s thyroiditis). His anti-TPO and anti-TG antibodies were very high (Anti-TPO: 600 IU/ml, Anti-TG: 243 IU/ml). His thyroid function tests were normal. And his glucose level was 166 mg/dl. The thyroid ultrasound revealed thin and thick fibrous bands, and patchy hypoechoic areas. He was recommended to be followed up by the endocrinology department. His hemogram and the other biochemical test results were normal and his hepatitis markers were negative. The patient was recommended a topical treatment with 4% hydroquinone and topical steroid. It was also recommended to change his oral antidiabetic for photosensitivity-inducing properties. The patient was informed about protection from sun.
Prior to the study, patient gave written consent to the examination and biopsy after having been informed about the procedure.
DISCUSSION
LPP is a hyperpigmentation disease first described in Indians by Bhutani et al. [5]. Although its etiology is unknown, exposure to sun, drugs, hepatitis C-virus, internal malignancies, disrupted carbohydrate metabolism, and henna and hair dyes have been suggested as etiological factors [4,6]. T-lymphocyte mediated cytotoxic activity has been blamed in its pathogenesis [6,7].
The mostly affected regions are the face and the neck. Upper extremities and torso may also be involved. Intertriginous lesions are less common [2]. It is generally asymptomatic, while itching may be observed [5]. The lesions are in the form of dark brown macules and papules. Scalp and nails remain unaffected and the oral mucosa is rarely involved. The pigmentation may be diffuse, reticular, speckled, unilaterally linear or perifollicular. The diffuse pattern is the most common pattern and the other forms are very rare [4]. In our patient, the lesions had a speckled pattern. Histopathologically, the epidermis was atrophied with vacuolar degeneration in the basal layer and lichenoid reaction characterized by infiltration in the dermis in the form of rare lymphocytic bands. Pigment incontinence and melanophages are typical. Also, erythema dyschromicum perstans, fixed drug reaction, postinflammatory hyperpigmentation, and contact dermatitis to the pigment should be considered in the differential diagnosis [2,6].
Hepatitis C, which is more closely associated with oral lichen planus, has been found to be positive in 60,6% of the patients with LPP in a study [4]. Ebrahimi et al. have observed at least one concurrent autoimmune disease in 33 (28%) of the patients with lichen planus [8]. Muzio et al. have detected Hashimoto’s thyroiditis in 15 among 105 patients (14.3%) with oral lichen planus and stated that this is a new association, that the thyroid autoantibodies in circulation triggers the organ-specific autoimmune response and leads to lichen planus lesions on the oral mucosa or the skin [9]. Susan et al. have found a significant correlation between autoimmune thyroiditis (15%), alopecia areata (%4), celiac disease (%2) and erosive lichen planus [10]. But, pigmented lichen planus associated with autoimmune diseases has not been reported.
There is no effective treatment for the condition. Potent topical steroids, calcineurin inhibitors, hydroquinone or retinoic acid may be tried. Spontaneous remission may be observed [3,7].
In patients who present with any kind of pigmentation on the face, lichen planus pigmentosus should be among the first diagnoses considered. Also, patients with lichen planus pigmentosus should be scanned for autoimmune diseases. According to our knowledge, our patient is the first case with pigmented lichen planus associated with diabetes and autoimmune thyroiditis.
Autoimmune diseases can affect the resistance to treatment, therefore new researches are necessary about this topic.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Nag F, Ghosh A, Chatterjee G, Choudhary N, Lichen planus pigmentosus: Two atyp?cal presentationOur Dermatol Online 2013; 4: 78-9.
2. Jung YJ, Lee YH, Lee SY, Lee WS, A case of lichen planus pigmentosus- inversus in a Korean patientAnn Dermatol 2011; 23: 61-3.
3. Zhang RZ, Zhu WY, One case of linear lichen planus pigmentosusOpen Dermatol J 2012; 6: 25-8.
4. Vachiramon V, Suchonwanit P, Thadanipon K, Bilateral linear lichen planus pigmentosus associated with hepatitis C virus infectionCase Rep Dermatol 2010; 2: 169-72.
5. Gaertner E, Elstein W, Lichen planus pigmentosus- inversus: case report and review of an unusual entityDermatol Online J 2012; 18: 11-4.
6. Kashima A, Tajiri A, Yamashita Y, Asada Y, Setoyama M, Two Japanese cases of lichen planus pigmentosus- inversusInt J Dermatol 2007; 46: 740-2.
7. Uyar B, Sivrikoz ON, A case of lichen planus pigmentosus- inversusTurkderm 2012; 46: 160-2.
8. Ebrahimi M, Lundqvist L, Wahlin YB, Nylander E, Mucosal lichen planus, a systemic disease requiring multidisciplinary care: a cross-sectional clinical review from a multidisciplinary perspectiveJ Low Genit Tract Dis 2012; 16: 377-80.
9. Lo Muzio L, Santarelli A, Campisi G, Lacaita M, Favia G, Possible link between Hashimoto’s thyroiditis and oral lichen planus: a novel association foundClin Oral Investig 2013; 17: 333-6.
10. Cooper SM, Ali Iaisha, Baldo M, Wojnarowska F, The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune diseaseArch Dermatol 2008; 144: 1432-5.
Notes
Source of Support: Nil
Conflict of Interest: None declared.
Comments are closed.