Intraepidermal and subepidermal blistering with skin necrosis, possibly caused by etanercept in treatment of a patient with psoriasis
Ana Maria Abreu Velez1, Billie L. Jackson1, Michael S. Howard2
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA, 2Billie L. Jackson, M.D., LLC; Dermatologist, Macon, Georgia, USA
ABSTRACT
Background: Etanercept is often used for treating patients with plaque psoriasis and psoriatic arthritis.
Case report: A 78 year old Caucasian female recently treated with Etanercept for plaque psoriasis developed necrotic patches and plaques on several areas of the body. Skin biopsies for hematoxylin and eosin (H&E) histology and immunohistochemistry(IHC), as well as direct immunofluorescence (DIF) were taken.
Results: H&E review revealed necrotic areas in the epidermis, with both intraepidermal and subepidermal blisters. Dilated dermal blood vessels with a perivascular infiltrate of lymphocytes, histiocytes and neutrophils were seen. DIF revealed anti-stratum corneum reactivity involving multiple immunoglobulins and complement factors, as well as deposits of fibrinogen and complement/C3c complexes at the basement membrane zone and in upper and intermediate dermal neurovascular areas. IHC review demonstrated an overexpression of von Willembrand factor, positive CEA in the stratum corneum. Focal BCL-10 and P53 overexpression on the basaloid and spinous layers of the epidermis was also noted. CD15 and myeloperoxidase expression were noted in the blisters as well as CD8 and CD45 expression around the upper dermal blood vessels.
Conclusions: We present a case of intraepidermal and subepidermal blistering with skin necrosis and document the presence of multiple immunoreactants in these lesions, possibly clinically linked to Etanercept.
Key words: Etanercept, blistering diseases, medication adverse events, severe adverse events, autoimmunity, necrosis, von Willembrand factor, vascular degeneration.
Abbreviations and acronyms: Immunohistochemistry (IHC), direct immunofluorescence (DIF), hematoxylin and eosin (H&E), tumor necrosis factor (TNF); basement membrane zone (BMZ), medication-related adverse drug reactions (ADRs), severe adverse events (SAEs).
INTRODUCTION
Enbrel© (Etanercept) is a biologic medical product that is used to treat autoimmune diseases by interfering with tumor necrosis factor (TNF; a soluble inflammatory cytokine) by acting as a TNF inhibitor [1]. Etanercept has U.S. FDA approval to treat rheumatoid, juvenile rheumatoid and psoriatic arthritis, plaque psoriasis and ankylosing spondylitis [1]. Etanercept is a fusion protein produced by recombinant DNA. It fuses the TNF receptor to the constant end of the IgG1 antibody [1]. On May 2, 2008, the FDA placed a black box warning on Etanercept, due to a number of complications associated with the drug in post-marketing reports of serious infections and sepsis, including fatalities. Tumour necrosis factor (TNF)-α is a proinflammatory cytokine that may induce anti-apoptotic proteins and endothelial cell activation factors in psoriasis, and therefore is commonly used in dermatology practices. It has also being proposed that Etanercept may inhibit neovascularisation in psoriatic lesions
CASE REPORT
A 78 year old Caucasian female had been recently treated with Etanercept for plaque psoriasis. A week later the patient presented excoriated, necrotic patches and plaques on the face, buttocks, chest, abdomen and extremities. Skin biopsies for hematoxylin and eosin (H&E) and immunohistochemistry (IHC) review, as well as direct immunofluorescence (DIF) were obtained. The Etanercept administration was halted, and topical steroids were given to the patient resulting in improvement of the necrotic lesions.
MATERIALS AND METHODS
For DIF, we incubated 4 micron thickness tissue sections on slides with secondary antibodies, as previously described [2–15]. We utilized FITC conjugated rabbit anti-total IgG (Dako, Carpinteria, California, USA) at dilutions of 1: 25. The samples were run with positive and a negative control. We also utilized FITC conjugated rabbit antisera to human IgG, IgA, IgM, IgD, IgE, complement/1q, complement/C3, fibrinogen and albumin. Our anti-human IgA antiserum (alpha chain) and anti-human IgM antiserum (Mu chain) were obtained from Dako. Our anti-human IgE antiserum (epsilon chain) was obtained from Vector Laboratories (Burlingame, California, USA). Our FITC conjugated anti-human IgD antibodies were obtained from Southern Biotechnology(Birmingham, Alabama, USA). The slides were then counterstained with 4’,6-diamidino-2-phenylindole(Dapi; Pierce, Rockford, Illinois, USA). Mouse anti-CD31/PECAM was utilized to stain dermal vessels (Invitrogen, Carlsbad, California, USA), and was specifically utilized with a secondary donkey anti-mouse IgG (heavy and light chains) conjugated with Alexa Fluor 555 (Invitrogen).
Immunohistochemistry (IHC)
We performed IHC utilizing multiple monoclonal and polyclonal antibodies, all from Dako (Carpinteria, California, USA). For all our IHC testing, we used a dual endogenous peroxidase blockage, with the addition of an Envision dual link to assist in chromogen attachment. We then applied the chromogen 3,3-diaminobenzidine(DAB), and counterstained with hematoxylin. The samples were run in a Dako Autostainer Universal Staining System. Positive and negative controls were consistently performed; our studies were specifically performed as previously described [2–15]. The following antibodies were utilized: monoclonal mouse anti-human vascular endothelial growth factor (VEGF) Clone VG1, monoclonal mouse anti-human BCL-10 protein, Clone 151, monoclonal mouse anti-human von Willebrand Factor, Clone F8/86, monoclonal mouse anti-human CD8, CD15, and CD45 and Myeloperoxidase (all from Dako), and Factor XIIIa (Novocastra, Chicago, Illinois, USA)).
RESULTS
Microscopic Description
A: Examination of the H&E tissue sections demonstrated focal, confluent parakeratosis within the stratum corneum. In addition, scattered collections of neutrophils were seen within the stratum corneum. Overall, the epidermis displayed minimal psoriasiform hyperplasia; the stratum granulosum was focally attenuated. Some focal epidermal spongiosis was seen. In focal areas, the epidermis was also ulcerated, with collections of neutrophils and serum scale crust present within the ulcer bases. Both intraepidermal and subepidermal blisters and clefts were seen. Dermal cellulitis is not present. Within the dermis, a mild, perivascular infiltrate of lymphocytes, histiocytes and neutrophils was present. Eosinophils were rare. No evidence of a neoplastic process was seen. Special stained slides were reviewed; the positive controls stained appropriately. The Gram special stain revealed collections of Gram positive, bacterial coccal organisms within the serum scale crust areas.
Direct immunofluorescence(DIF)
Demonstrated the following results: IgG (+, diffuse epidermal stratum corneum); IgA (+, diffuse epidermal stratum corneum); IgM (+, diffuse epidermal stratum corneum); IgD (+++, diffuse epidermal stratum corneum); IgE (+, Lower papillary dermal perineural); Complement/C1q(-); Complement/C3 (+, diffuse epidermal stratum corneum and blood vessels in the middle dermis); Complement/C4 (+, diffuse epidermal stratum corneum); CD31/PECAM(+, overexpressed dermal vascular endothelial); Kappa light chains (+, diffuse epidermal stratum corneum); Lambda light chains(+, diffuse epidermal stratum corneum) albumin (+, diffuse epidermal stratum corneum) and fibrinogen (+++, diffuse epidermal stratum corneum, and focal linear shaggy basement membrane zone (BMZ) (Fig. 1).
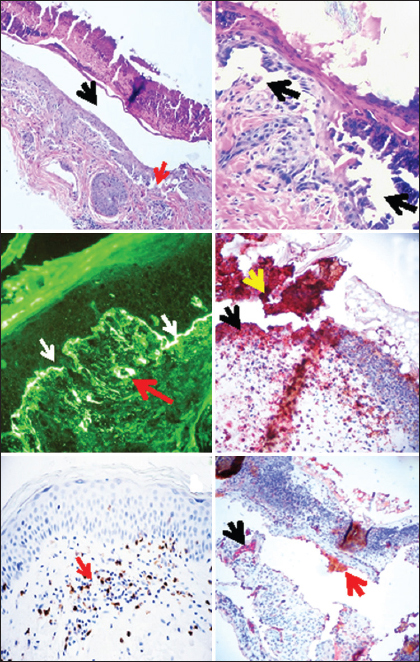
Figure 1: (a and b) H & E stain showing the epidermal corneal layer with necrosis and a subcorneal blister (black arrow), and also some subepithelial blisters (red arrows), (100X), In b, higher magnification of a, showing the subepithelial blisters (black arrows) (200X). c. DIF, using anti-human FITC conjugated fibrinogen antibody positivity, with pseudo-basement membrane deposits in a serrated manner (white staining; white arrows) as well as staining against the upper dermal blood vessels (white staining; red arrow). d. IHC double staining, using anti-human CD15 in brown and anti-CD45 in red. The photomicrograph highlights an amalgamation of the positivity of both markers in the crust (yellow arrow), as well as on the blister floor (black arrow). e. IHC staining with CD8 antibody, showing positive staining in an upper dermal perivascular infiltrate (brown staining; red arrow). f. IHC double staining, using anti-human myeloperoxidase in brown and anti-von Willembrand factor in red. The photomicrograph highlights damage to dermal blood vessels and (red staining; black arrow) and the presence of myeloperoxidase in the crust(brown staining; black arrow).
Immunohistochemistry (IHC)
As expected, myeloperoxidase and CD15 were very positive in the blister, around the edges of the blisters and in the crust material. CD45 was positive in some areas of the epidermal corneal layer, within the blisters and around upper dermal blood vessels. CD8 was positive mainly around the upper dermal inflammatory infiltrate. In the upper and intermediate dermal blood vessels, some markers such VEGF and von Willembrand factor seemed to be overexpressed. We also noted that P53 and BCL-10 were positive in the epidermis, but with strong expression around the blister edges and in some focal BMZ areas. Factor XIIIa was positive around the edges of the blisters (Fig. 1).
DISCUSSION
Etanercept has been successfully utilized in the treatment of multiple diseases, including psoriasis and autoimmune skin blistering diseases; however, multiple secondary reactions involving the skin and other organs have been documented [16–29]. Some adverse reactions and conditions include primary thyroid marginal zone B-cell lymphoma, sinusitis, herpes zoster, herpes simplex, antiphospholipid antibodies, cerebral ischemic damage, hypertension, carcinoma cuniculatum (verrucous squamous cell carcinoma), urticaria, angioedema-like reactions, nodular fasciitis,Coxiella burnetii infection(Q fever), erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, tuberculosis, apoptotic enthreopathy, pyogenic granuloma, alopecia, lichen striatus, alterations in sperm quality, a possible cerebral venous thrombosis, lymphopenia, neurosarcoidosis, systemic sarcoidosis, lichen planus, acute transverse myelitis, rosacea, salmonella septic arthritis, Staphylococcus aureuscolonization and injection site reactions[16–29]. Other documented adverse reactions include generalized pustular psoriasis, for which a differential diagnosis of acute generalized examthematous pustulosis(AGEP) should be considered.
Multiple autoimmune diseases and syndromes have also been reported to develop after the use of TNF-alpha inhibitors including vasculitis, lupus erythematosus and interstitial lung diseases [16–29]. Some studies have also shown that a predisposing sensitivity of patients to TNF-alpha inhibitors could be genetically determined, and may be due to genetic polymorphisms in selected genes. Some authors have demonstrated seroconversion with development of positive anti-ANA/dsDNA of the IgG subtype in patients receiving TNF-alpha inhibitors, as well as describing the onset of lupus/vasculitis [16–29]. Autoimmune induced renal disorders have also been described following TNF-alpha inhibitor use, including glomerulonephritis associated with systemic vasculitis, glomerulonephritis in a lupus-like syndrome and isolated autoimmune renal disorders [16–29].
Our findings indicate that Etanercept did possibly trigger the patient’s secondary blistering and ulceration reaction due to involvement of the dermal vasculature. Moreover, this medication could not stop the anti-corneal antibodies that were still being seen on the DIF. Given the fact that we observed some superinfection with Gram positive cocci in the ulcers, Etanercept could have some immunosupressant role in the psoriatic plaques. Other notable observations are that the BCL10 and P53 markers seem to play some role in development of the blisters, possibly by some apoptotic regulatory role that remains unknown.
Given our data, it is important to consider that there are other anti-TNF monoclonal antibodies similar to Etanercept, including Infliximab®, Adalimumab®, Certolizumab®, Golimumab® and Pegsunercep®. Thus, these medications may act as putative triggers in some secondary reactions in patients treated for plaque psoriasis.
CONCLUSIONS
We report an unknown reaction, possibly triggered by Etanercept and featuring intraepidermal and subepidermal blistering with skin necrosis. The incidences of inflammatory skin reactions due and/or related to Etanercept need to be considered when patients are using this medication suddenly develop dermatologic side effects, including those described here.
CONSENT
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexateN Engl J Med 1999; 340: 253-9.
2. Abreu Velez AM, Googe PB, Howard MS, In situ immune response in skin biopsies from patients affected by autoimmune blistering diseasesOur Dermatol Online 2013; 4: Suppl.3606-12.
3. Abreu Velez AM, Calle-Isaza J, Howard MS, CD1a, HAM56, CD68 and S-100 are present in lesional skin biopsies from patients affected by autoimmune blistering diseasesOur Dermatol Online 2014; 5: 113-7.
4. Abreu Velez AM, Calle-Isaza J, Howard MS, HLA-DPDQDR is expressed in all lesional skin from patients with autoimmune skin diseasesOur Dermatol Online 2014; 5: 125-8.
5. Abreu Velez AM, Googe PB, Howard MS, Ribosomal protein s6-ps240 is expressed in lesional skin from patients with autoimmune skin diseasesNorth Am J Med Sci 2013; 5: 10604-8.
6. Abreu Velez AM, Calle-Isaza J, Howard MS, A case of bullous pemphigoid with immunoreactivty to blood vessels and sweat glandsOur Dermatol Online 2013; 4: Suppl.3621-4.
7. Abreu Velez AM, Googe PB, Howard MS, Immunohistochemistry versus immunofluoresence in the diagnosis of autoimmune blistering diseasesOur Dermatol Online 2013; 4: Suppl.3627-30.
8. Abreu Velez AM, Smoller BR, Howard MS, Rouleaux and autoagglutination of erythrocytes associated with fibrin-like material in skin biopsies form patients with autoimmune blistering diseasesOur Dermatol Online 2013; 4: Suppl.3613-5.
9. Abreu Velez AM, Roselino AM, Howard MS, Mast cells, Mast/Stem Cell Growth Factor receptor (c-kit/cd117) and IgE may be integral to the pathogenesis of endemic pemphigus foliaceusOur Dermatol Online 2013; 4: Suppl.3596-600.
10. Abreu Velez AM, Calle-Isaza J, Howard MS, Cyclo-oxygenase 2 is present in the majority of lesional skin from patients with autoimmune blistering diseasesOur Dermatol Online 2013; 4: 476-8.
11. Abreu Velez AM, Yepes-Naranjo MM, Avila IC, Londoño ML, Googe PB, Velásquez-Velez JE, Velez ID, Tissue inhibitor of metalloproteinase 1, Matrix metalloproteinase 9, alpha-1 antitrypsin, metallothionein and urokinase type plasminogen activator receptor in skin biopsies from patients affected by autoimmune blistering diseasesOur Dermatol Online 2013; 4: 275-80.
12. Abreu Velez AM, Yi H, Girard JG, Zhe J, Duque-Ramírez M, Arias LF, Dermatitis herpetiformis bodies and autoantibodies to noncutaneous organs and mitochondria in dermatitis herpetiformisOur Dermatol Online 2012; 3: 283-91.
13. Abreu Velez AM, Brown VM, Howard MS, Cytotoxic and antigen presenting cells present and non-basement membrane zone pathology in a case of bullous pemphigoidOur Dermatol Online 2012; 3: 93-9.
14. Abreu Velez AM, Girard JG, Howard MS, IgG bullous pemphigoid with antibodies to IgD, dermal blood vessels, eccrine glands and the endomysium of monkey esophagusOur Dermatol Online 2011; 2: 48-51.
15. Abreu Velez AM, Smith JG, JrHoward MS, IgG/IgE bullous pemphigoid with CD45 lymphocytic reactivity to dermal blood vessels, nerves and eccrine sweat glandsNorth Am J Med Sci 2010; 2: 538-541.
16. Shetty A, Marcum CB, Glass LF, Carter JD, Successful treatment of pemphigus vulgaris with etanercept in four patientsJ Drugs Dermatol 2009; 8: 940-3.
17. Beuthien W, Mellinghoff HU, von Kempis J, Skin reaction to adalimumabArthritis Rheum 2004; 50: 1690-2.
18. Lecluse LL, Dowlatshahi EA, Limpens CE, de Rie MA, Bos JD, Spuls PI, Etanercept: an overview of dermatologic adverse eventsArch Dermatol 2011; 147: 79-94.
19. Borrás-Blasco J, Gracia-Perez A, Rosique-Robles JD, Nuñez-Cornejo C, Casterá MD, Abad FJ, J Urticaria due to etanercept in a patient with psoriatic arthritisSouth Med J 2009; 102: 304-5.
20. Kardaun SH, Kuiper H, Fidler V, Jonkman MF, The histopathological spectrum of acute generalized exanthematous pustulosis (AGEP) and its differentiation from generalized pustular psoriasisJ Cutan Pathol 2010; 37: 1220-9.
21. Fujikawa K, Kawakami A, Hayashi T, Iwamoto N, Kawashiri SY, Aramaki T, Cutaneous vasculitis induced by TNF inhibitors: a report of three casesMod Rheumatol 2010; 20: 86-9.
22. Gómez-Mateo C, Avalos-Peralta SP, Ríos-Martín JJ, Carrizosa-Esquivel AM, González-Cámpora R, Camacho-Martínez F, [Sequential histological and immunohistochemical assessment of proliferation and apoptotic markers during treatment of psoriasis with antitumor necrosis factor alpha (infliximab)]Actas Dermosifiliogr 2009; 100: 420-4.
23. Béné J, Moulis G, Auffret M, Lefevre G, Coquerelle P, Coupe P, Alopecia induced by tumour necrosis factor-alpha antagonists: description of 52 cases and disproportionality analysis in a nationwide pharmacovigilance databaseRheumatology (Oxford) 2014; 53: 1465-9.
24. Usero-Ruiz M, Díaz-Sánchez M, Moniche-Álvarez F, [Etanercept: does it constitute a risk factor for development of cerebral venous thrombosis?]Rev Neurol 2014; 1: 58239-40.
25. Pepper AN, Talreja N, Cowan GM, Glaum MC, Lockey RF, Lymphopenia induced by etanerceptAnn Allergy Asthma Immunol 2014; 112: 262-3.
26. Varley CD, Deodhar AA, Ehst BD, Bakke A, Blauvelt A, Vega R, Persistence of Staphylococcus aureus colonization among individuals with immune-mediated inflammatory diseases treated with TNF-a inhibitor therapyRheumatology (Oxford) 2014; 53: 332-7.
27. Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 casesMedicine (Baltimore) 2007; 86: 242-51.
28. Takase K, Horton SC, Ganesha A, Das S, McHugh A, Emery P, What is the utility of routine ANA testing in predicting development of biological DMARD-induced lupus and vasculitis in patients with rheumatoid arthritis? Data from a single-centre cohortAnn Rheum Dis 2014; 73: 1695-9.
29. Piga M, Chessa E, Ibba V, Mura V, Floris A, Cauli A, Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: systematic literature review and analysis of a monocentric cohortAutoimmun Rev 2014; 13: 873-9.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Comments are closed.