Anti-thyroglobulin Antibody and Vitiligo: A Controlled Study
Emina Kasumagic-Halilovic, Nermina Ovcina-Kurtovic, Hana Helppikangas
Department of Dermatovenereology, Sarajevo University Clinical Center, Bolnička 25, 71 000 Sarajevo, Bosnia and Herzegovina
ABSTRACT
Introduction: Vitiligo is an acquired skin disorder characterized by depigmented maculae resulting from a reduction of the number and function of melanocytes. The etiopathogenesis of the disease is still unclear, but there is evidence that autoimmunity and endocrine disfunction may be involved.
Objective: The aim of this study was to evaluate serum levels of anti-thyroglobulin antibody (anti-Tg) in vitiligo patients and control subjects, and also to assess the difference between the localized and generalized forms of the disease.
Methods: In this prospective study we investigated serum level of anti-Tg in 33 patients with vitiligo and 33 healthy controls. We also examined a possible association between serum levels of anti-Tg and disease severity.
Results: Comparison of median values of anti-Tg has showed that serum concentrations of anti-Tg are significantly higher (p<0.05) in serum samples of vitiligo patients in relation to control group. Statistically significant difference was also found in values of anti-Tg between patients with generalized and patients with localized vitiligo (p<0.05).
Conclusion: This study shows a significant association between vitiligo and thyroid autoimmunity, and that tests to detect anti-Tg are relevant in patients with vitiligo.
Key words: Vitiligo; thyroid autoimmunity; anti-thyroglobulin antibody
INTRODUCTION
Vitiligo is a chronic skin disorder characterized by progressive loss of functional melanocytes, which results in depigmentated macules in skin, hair and mucous membrane. It affects approximately 1% of the general population, with the disease beginning before the age of 20 in 50% of cases [1]. The disease is classified according to its extent and distribution, and can be subdivided into generalised or localised. In both types, the melanocytes are destroyed, resulting in loss of pigment from circumscribed areas of the skin. Although several theories have been proposed to explain the loss of melanocytes in vitiligo, the etiopathogenesis of the disease is still unclear. The clinical association with autoimmune disorders and organ specific antibodies indirectly support the idea of an autoimmune pathogenesis of the disease. In addition, many studies have indicated a role for both cellular and humoral immunity in the pathogenesis of vitiligo [2,3]. At the site of depigmentation, T cell infiltrates are invariably seen in patients with active vitiligo, along with a high frequency of cytotoxic T lymphocytes specific for melanocytic antigens, suggesting a direct melanocyte specific T cell attack [4]. Furthermore, many patients with vitiligo have serum auto antibodies directed against melanocytes. How antibodies to pigment cells arise, in vitiligo patients, has not yet been elucidated. They might result from a genetic predisposition to immune dysregulation at T or B cell levels. Involvement of the immune system in the pathogenesis is evidenced by the effectiveness of immunomodulatory agents, such as, corticosteroids and calcineurin inhibitors [5].
In 1956, anti-thyroglobulin antibody (anti-Tg) was first demonstrated as an auto antibody in the serum of patients with Hashimoto’s thyroiditis [6], and this finding first established the concept of organ-specific autoimmune disease. It has been known for some time that anti-Tg shows direct cytotoxic and cell destruction activity and maintain the thyroid autoimmune process over time, promoting the presentation of anti-Tg to T-cells by B-cells with antigen-presenting function [7]. Anti-Tg also promotes the proliferation of CD4-positive B- and T-lymphocytes in response to thyroglobulin [8]. These effects seem to be directly correlated with autoantibody concentration, and open up a new scenario regarding the role of B-cells with antigen-presenting function, in autoimmune thyroid disease, and new therapeutic possibilities for autoimmune diseases.
In the past, several authors described an association of vitiligo with autoimmune disorders and the presence of different tissue auto antibodies. A review of the literature showed large differences in the results. Therefore, the aim of our study was to evaluate serum levels of anti-Tg in patients with vitiligo and healthy subjects, and also to assess a possible association between anti-Tg and clinical type of the disease.
METHODS
The study is a clinical cross-sectional study carried out among patients with vitiligo in the Department of dermatology at the Sarajevo University Clinical Center. The study included 33 patients with vitiligo, 20 female and 13 male, median age 34.67 (±14.74) years. Of them, there were 14 (42.4%) patients with generalized vitiligo (GV) and 19 (57.6%) patients with localized form of disease (LV). A detailed history and examination were undertaken in all study subjects, including patients’ age, age at onset, duration of disease, and the severity of disease. The diagnosis of vitiligo was made on clinical grounds. Skin biopsy was performed in selected cases. Patients with depigmenting disorders other than vitiligo were excluded. The control group consisted of 33 volunteers, 19 female and 14 male, median age 40.33 (±14.78) years. Blood samples were taken and a physical examination was performed. All subjects gave their informed consent in accordance with the requirements of the institutional Ethics Committee. The study was conducted in accordance with the Declaration of Helsinki.
Serum levels of anti-Tg (threshold value: 115 IU/ml) were measured by use of electrochemiluminiscence immunoassay (ECLIA) accorsing to standard protocols (COBAS, Roche Diagnostics GmbH, Germany).
Statistics
Analysis was carried out by calculating 95% confidence interval (95%CI) for median values of anti-Tg (IU/ml). The distribution of laboratory values anti-Tg were compared between groups using Mann-Whitney test. Values with P<0.05 were accepted as statistically significant. We used the point biserial correlation coefficient (rpb) for analysis of the relationship between dichotomous variable (vitiligo and control) and continuous variable (anti-Tg). Statistical significance was set at P<0.05.
Statistical analyses were performed using MedCalc for Windows, version 11.4.1.0 (MedCalc Software, Mariakerke, Belgium).
Ethics
This study was performed on human subjects; thus, all patients were aware of the presence of the study and they were fully informed about the study.
All subjects gave their informed consent and ethical clearance was obtained from local ethical committee.
RESULTS
We performed a cross-sectional study in 33 consecutive patients with vitiligo and 33 sex- matched controls. The mean age of onset (SD) was 37.74 (12.45) years. The duration of vitiligo ranged from 1 to 252 months, the mean duration (SD) was 55.85 (66.24). Fourteen (42.4%) patients had generalized, and nineteen (57.6%) patients had localized vitiligo. The most commonly involved site was the face in 15 (45.5%), followed by upper limbs in 10 (30.3%) and lower limbs in 8 (24.2%) patients.
In patients with vitiligo anti-Tg antibody titers were ranging from 10.5 to 1021 IU/mL, with the highest values observed in the GV patients. In control group anti-Tg antibody titers were ranging from 5.1 to 129 IU/mL.
Mann-Whitney test found statistically significant difference in anti-Tg (IU/ml) between Vitiligo group (VG) (Md=31.000, n=33, 95%CI=15.441-92.382) and Control group (CG) (Md=16.300, n=33, 95%CI=12.941-35.263), z=2.334, P=0.0196 (Table 1, Fig. 1).
Test point biserial coefficient of correlation showed that more values of anti-Tg correlated moderately rpb=0.290 (P=0.018) with vitiligo.
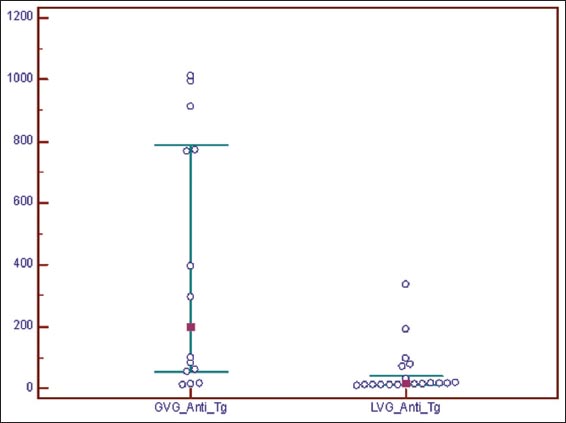
Mann-Whitney test found statistically significant difference in anti-Tg (IU/ml) between Generalized vitiligo group (GVG) (Md=198.050, n=14, 95%CI=51.549-787.699) and Localized vitiligo group (LVG) (Md=15.500, n=19, 95%CI=12.978-39.918), z=3.023, P=0.0025 (Table 2, Fig. 2).
DISCUSSION
Known for thousands of years because of its visually evident phenotype, vitiligo is the most common pigmentary disorder [9]. Despite its long history, our knowledge is actually limited. Several hypothesis have been proposed to explain the pathogenesis of vitiligo and indeed it is likely that more than one mechanism is responsible for the clinical manifestations of the disease [10]. Autoimmunity is one hypothesis forwarded to explain vitiligo aetiology.
Vitiligo has been reported in association with numerous autoimmune disorders and the presence of different tissue antibodies. One of the main associations is the one marked with thyroid abnormalities. Both clinical and subclinical thyroid diseases have been mentioned to be more common in patients with vitiligo, as compared with healthy subjects. It was already in 1941, when Robert suggested that vitiligo might be connected with an increased activity of the thyroid gland [11]. He noted a distinct rise of the basal metabolism in 10 out of 20 vitiligo patients tested. Several authors reported a significantly increased prevalence of autoimmune thyroid disease in vitiligo patients, while the rate of positivity of thyroid auto antibodies varied from 2.2% [12] to 82% [13]. In addition, there is also a study reporting a significantly increased prevalence of vitiligo in patients with autoimmune thyroid disease compared to patients with non autoimmune thyroid disease [14,15]. The risk for patients with vitiligo to develop thyroid diseases is almost twice as high, when compared to patients without vitiligo, and the risk of elevated thyroid antibodies with vitiligo is more than five-fold higher in comparison with patients without vitiligo [16]. It is possible that the occurrence of these diseases in the same patient is the result of the basic autoimmune disturbance involving the melanocytes, and the thyroid glands in a patient who is genetically predisposed to these disease. Both diseases shows alteration of T-cell population and this can be explained with common genetic background of their autoimmunity. Spritz demonstrated inherited susceptibility to generalized vitiligo involves a number of specific genes, many of which are shared with other autoimmune diseases that are epidemiologically associated with vitiligo, including autoimmune thyroid diseases [17,18].
In accordance to previous studies, we also demonstrated that anti-thyroglobulin antibody was significantly increased in vitiligo patients in comparison to healthy subjects. The patients with generalized vitiligo have higher occurrences of both thyroid disease and thyroid antibodies, compared with localized vitiligo. This is in accordance with the assumption that the susceptibility for autoimmune disease concerns generalized vitiligo more than localized vitiligo [19]. In our study, statistically significant difference was also found in values of anti-Tg between patients with generalized and patients with localized vitiligo.
The nature of the relationship between vitiligo and thyroid autoimmunity is presently unknown. Possible explanations for the relationship of these autoimmune diseases include: (1) immunomodulatory effects of antithyroid antibodies, (2) molecular mimicry between thyroid and disease-specific epitopes, and (3) genetic linc between thyroid autoimmunity and the susceptibility to autoimmune disease [20]. It is a multidisciplinary problem requiring cooperation of specialist in different fields of medicine. Both dermatologist and endocrinologists have to inquire their patients about the family history of autoimmune diseases and to look for associated autoimmune disorders.
CONCLUSION
Vitiligo often precedes thyroid dysfunction by many years. Therefore, the presence of elevated thyroid antibodies may serve as useful clinical tool in euthyroid subjects with vitiligo to identify patients at risk for thyroid disease. The increased frequency of autoimmune diseases, in patients with vitiligo, suggests that all these conditions share a common etiologic factor.
REFERENCES
1. Alikhan A, Felsten LM, Daly M, Petronic-Rosic V, Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, associations, histopathology, etiology, and work-upJ Am Acad Dermatol 2011; 65: 473-91.
2. Sanchez-Sosa S, Aquirre-Lombardo M, Jimenez-Brito G, Ruiz-Arguelles A, Immunophenotypic characterization of lymphoid cell infiltrates in vitiligoClin Exp Immunol 2013; 173: 179-83.
3. Rezaei N, Gavalas NG, Weetman AP, Kemp AH, Autoimmunity as an aethiological factor in vitiligoJEADV 2007; 21: 865-76.
4. Sharquie KE, Mehenna SH, Naji AA, Al-Azzawi H, Inflammatory changes in vitiligo: stage I and II depigmentationAm J Dermatopathol 2004; 26: 108-12.
5. Glassman SJ, Vitiligo, reactive oxygen species and T-cellsClin Sci (Lond) 2011; 120: 99-120.
6. Roitt IM, Doniach D, Campbell RN, Hudson RV, Autoantibodies in Hashimoto’s disease (lymphadenoid goitre)Lancet 1956; 2: 820.
7. Tozzoli R, Villata D, Kodermaz G, Bagnasco M, Tonutti E, Bizzaro N, Autoantibody profiling of patients with autoimmune thyroid disease using a new multiplex immunoassay methodClin Chem Lab 2006; 44: 837-42.
8. Nielsen CH, Hegedus L, Leslie RG, Autoantibodies in autoimmune thyroid disease promote immune complex formation with self antigens and increase B cell and CD4+cell proliferation in response to self antigensEur J Immunol 2004; 34: 263-72.
9. Spitz RA, The genetic of generalized vitiligo and associated autoimmune diseasesPigment Cell Res 2007; 20: 271-78.
10. Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachandran AV, Vitiligo: interplay between oxidative stress and immune systemExp Dermatol 2013; 22: 245-50.
11. Robert P, Ueber die VitiligoDermatologica 1941; 84: 317-90.
12. Al-Mutairi N, Sharma A, Profile of vitiligo in Farwaniya region in KuwaitKuwait Med J 2006; 38: 128-31.
13. Kumar KV, Priya S, Sharma R, Kapoor U, Saini M, Bisht YS, Autoimmune thyroid disease in patients with vitiligo: prevalence study in IndiaEndocr Pract 2012; 18: 194-99.
14. Ingordo V, Gentile C, Iannazzone SS, Cusano F, Naldi L, Vitiligo and autoimmunity: an epidemiological study in a representative sample of young Italian malesJEADV 2011; 25: 105-9.
15. Uncu S, Yayli S, Bahadir S, Okten A, Alpay K, Relevance of autoimmune thyroiditis in children and adolescents with vitiligoInt J Dermatol 2011; 50: 175-9.
16. Vrijman C, Kroon MW, Limpens J, The prevalence of thyroid disease in patients with vitiligo: a systematic reviewBr J Dermatol 2012; 167: 1224-35.
17. Spritz RA, Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid diseaseThyroid 2010; 20: 745-54.
18. Spritz RA, The genetic of generalized vitiligo and associated autoimmune diseasesPigment Cell Ress 2007; 20: 271-78.
19. Laberge G, Mailloux CM, Gowan K, Holland P, Bennet DC, Fain PR, Early disease onset and increased risk of other autoimmune diseases in familiar generalized vitiligoPigment Cell Res 2005; 18: 300-5.
20. Szyper-Kravitz M, Marai I, Shoenfeld Y, Coexistence of thyroid autoimmunity with other autoimmune diseases: friend or foe? Additional aspects on the mosaic of autoimmunityAutoimmunity 2005; 38: 247-55.
Notes
Source of Support: Nil
Conflict of Interest: None declared.

Comments are closed.