Our Dermatol Online. 2013; 4(2): 208-211
DOI:. 10.7241/ourd.20132.50
Date of submission: 01.02.2013 / acceptance: 03.03.2013
Conflicts of interest: None
OCULOCUTANEOUS ALBINISM COMPLICATED WITH AN ULCERATED PLAQUE
Belliappa Pemmanda Raju, Umashankar Nagaraju, Leena Raveendra, Vivekananda, Priya Kootelu Sundar, Lokanatha Keshavalu
Department of Dermatology, Rajarajeswari Medical College and Hospital, Kambipura, Kengeri Hobli, Mysore Road, Bangalore 560074, Karnataka, India
Corresponding author: Ass. Prof. Belliappa Pemmanda Raju e-mail: drbelliappa@gmail.com
How to cite an article: Raju BP, Nagaraju U, Raveendra L, Vivekananda, Sundar PK, Keshavalu L. Oculocutaneous albinism complicated with an ulcerated plaque. Our Dermatol Online. 2013; 4(2): 208-211.
Abstract
A 32-year-old male with a history of albinism and farmer by occupation presented with an ulcerated plaque on the right wrist. The patient had light eyes, hair, and skin. Physical examination showed extensive photodamage. A skin biopsy specimen from the plaque revealed a well-differentiated squamous-cell carcinoma. Wide surgical excision was done. The most common types of oculocutaneous albinism (OCA), OCA 1 and OCA 2, are autosomal recessive disorders of pigmentation that commonly affect the skin, hair and eyes. Photodamage and skin cancers plague patients with albinism. Albinos face a myriad of social and medical issues. Importance of photoprotection, skin cancer surveillance and treatment has been stressed upon in this report.
Key words: albinism; photoprotection; melanin; squamous cell carcinoma
Introduction
Albinism is a genetically inherited disorder characterized by hypopigmentation of the skin, hair and eyes due to a reduced or lack of cutaneous melanin pigment production [1]. Generally, there are two principal types of albinism, oculocutaneous, affecting the eyes, skin and hair, and ocular affecting the eyes only [1,2]. The mode of inheritance of albinism is thought to vary, depending on the type. The oculocutaneous type is considered autosomal recessive, and the ocular variant sex-linked [1-3]. Ocular problems faced by albinos are nystagmus, strabismus, photophobia, foveal hypoplasia and decreased visual acuity. The cutaneous problems seen with oculocutaneous albinism include sunburns, blisters, centro-facial lentiginosis, ephelides, solar elastosis, solar keratosis, basal cell carcinomas and squamous cell carcinomas. Squamous cell carcinoma has been reported to be the commonest skin malignancy seen in albinos [4,5]. Albinos are at an increased risk of developing skin malignancies due to the absence of melanin, which is a photo protective pigment, protecting the skin from the harmful effects of ultraviolet radiation [6]. Hence, a regular examination for early detection and treatment of these malignancies would increase their life expectancy to a great extent. We report here a case of oculocutaneous albinism with well-differentiated squamous cell carcinoma in a farmer and also review oculocutaneous albinism with emphasis on treatment and preventive aspects.
Case Report
A 32- year- old male, who was a known case of Oculocutaneous Albinism, presented with an ulcerated lesion over the right forearm. It started as a small wound which arised from normal looking skin and gradually increased to the present form over a period of six months. The patient was a farmer who had occupational sun exposure with no apparent photoprotection for the past fifteen years. History of photosensitivity and photophobia was present. He was born out of a non-consanguineous marriage. He had an elder sibling who was unaffected, but had an affected first-degree relative. On physical examination, generalized depigmented skin with white hairs, brownish freckles and telangiectasia were seen on his body (Fig. 1 – 3). Ocular Examination revealed photophobia, nystagmus and decreased visual acquity. Ulcerated plaque measuring 4cm x 3 cm with crusting with rolled out edges was seen on the right wrist (Fig. 4a, b). It was fixed to the underlying tissues. Regional lymphadenopathy was absent. General physical and systemic examination was normal. On the basis of these clinical findings, a differential diagnosis of Oculocutaneous Albinism with Squamous Cell Carcinoma (SCC)/ Basal Cell Carcinoma (BCC)/ Malignant Melanoma (MM) was made. Routine haematological and biochemical investigations were normal. Chest X-Ray and Ultrasound Abdomen did not reveal any abnormalities. Biopsy from the ulcerated plaque showed features of well differentiated squamous cell carcinoma (Fig. 5). Wide surgical excision was done in this patient and counselled regarding photoprotection. He is under long term follow-up for recurrences.
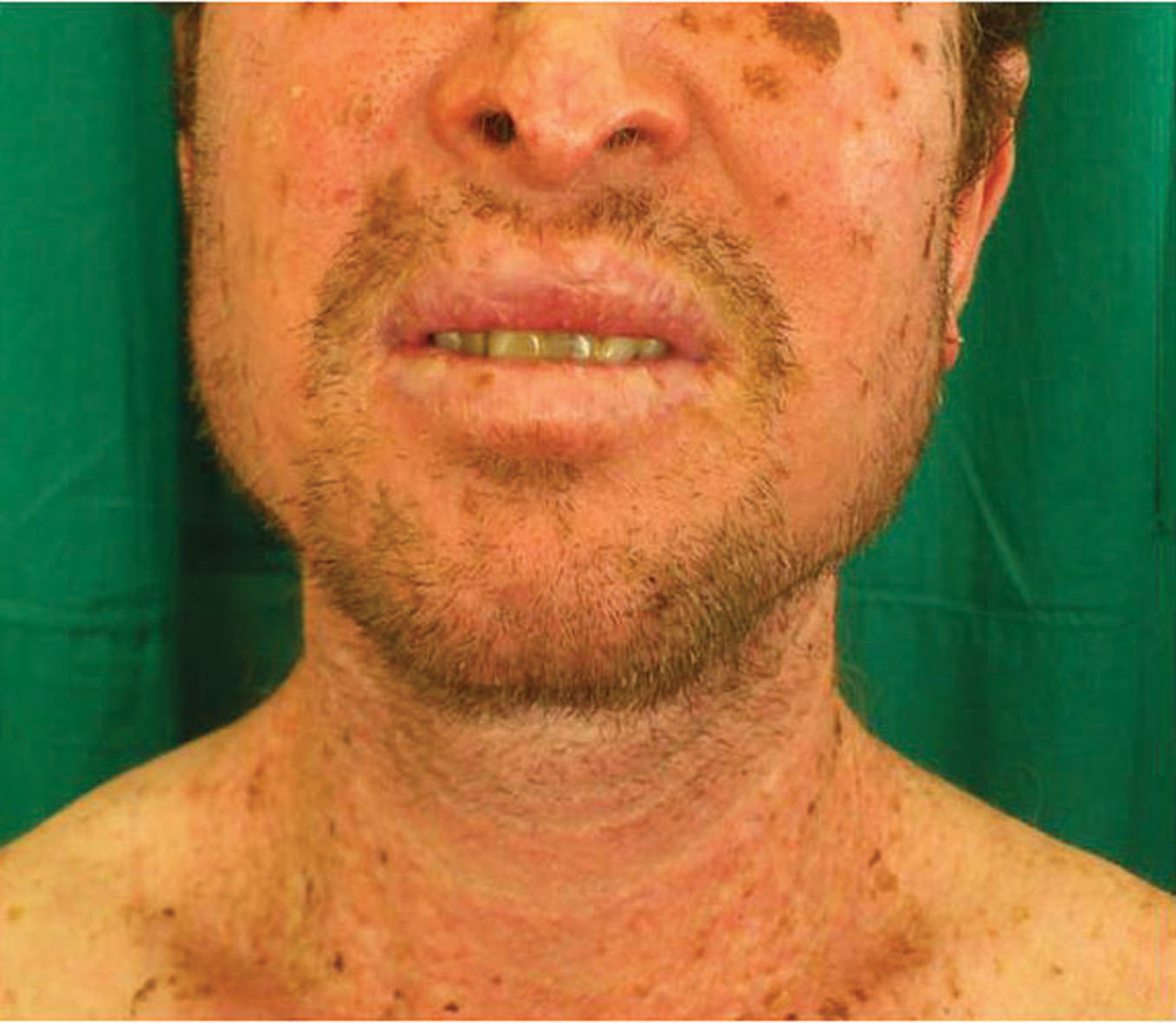 Figure 1. Generalized depigmented skin with white hairs, brownish freckles and telangiectasia
|
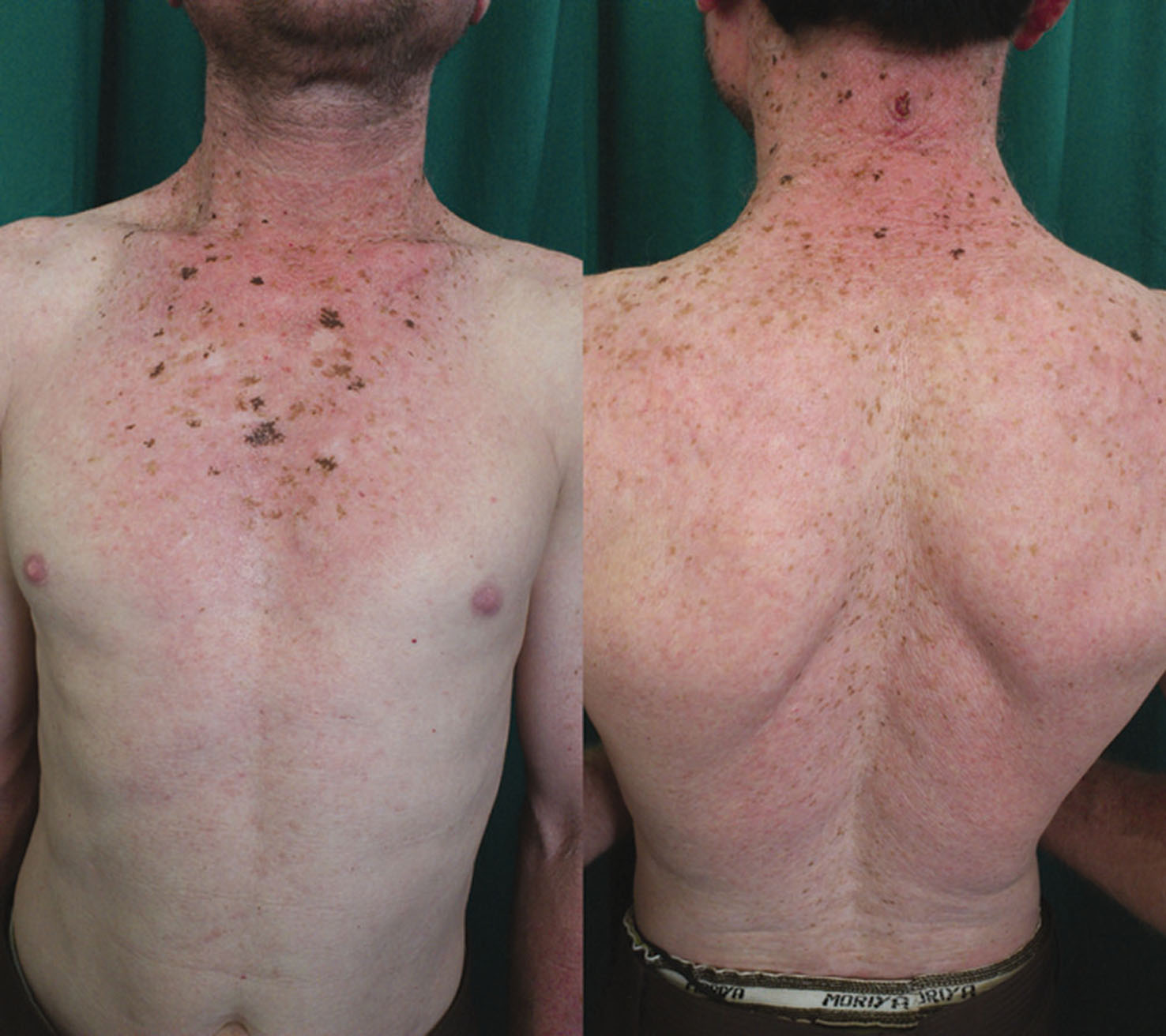 Figure 2 and 3. Depigmentation with brownish freckles and telangiectasia over the chest and back
|
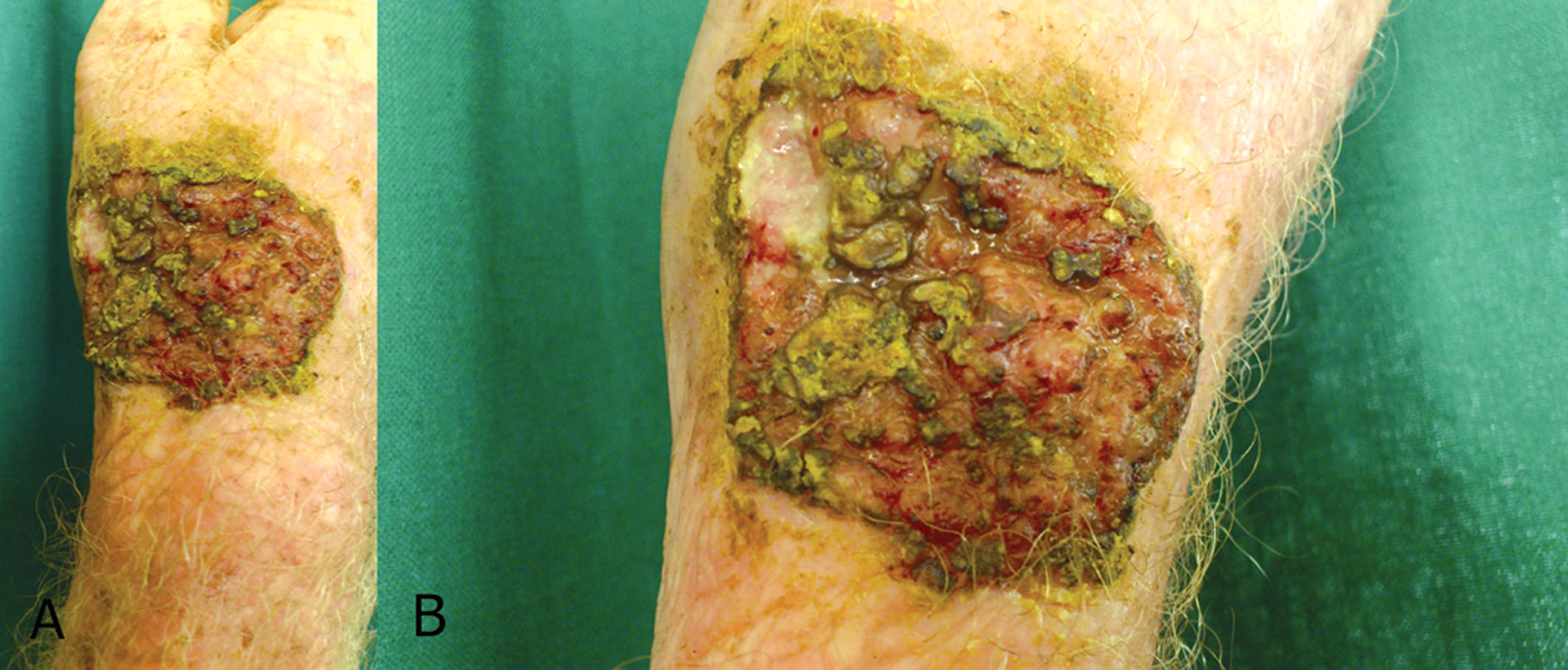 Figure 4A. Ulcerated plaque with crusting and rolled out edges on the right wrist. B. Close-up view of the ulcerated plaque
|
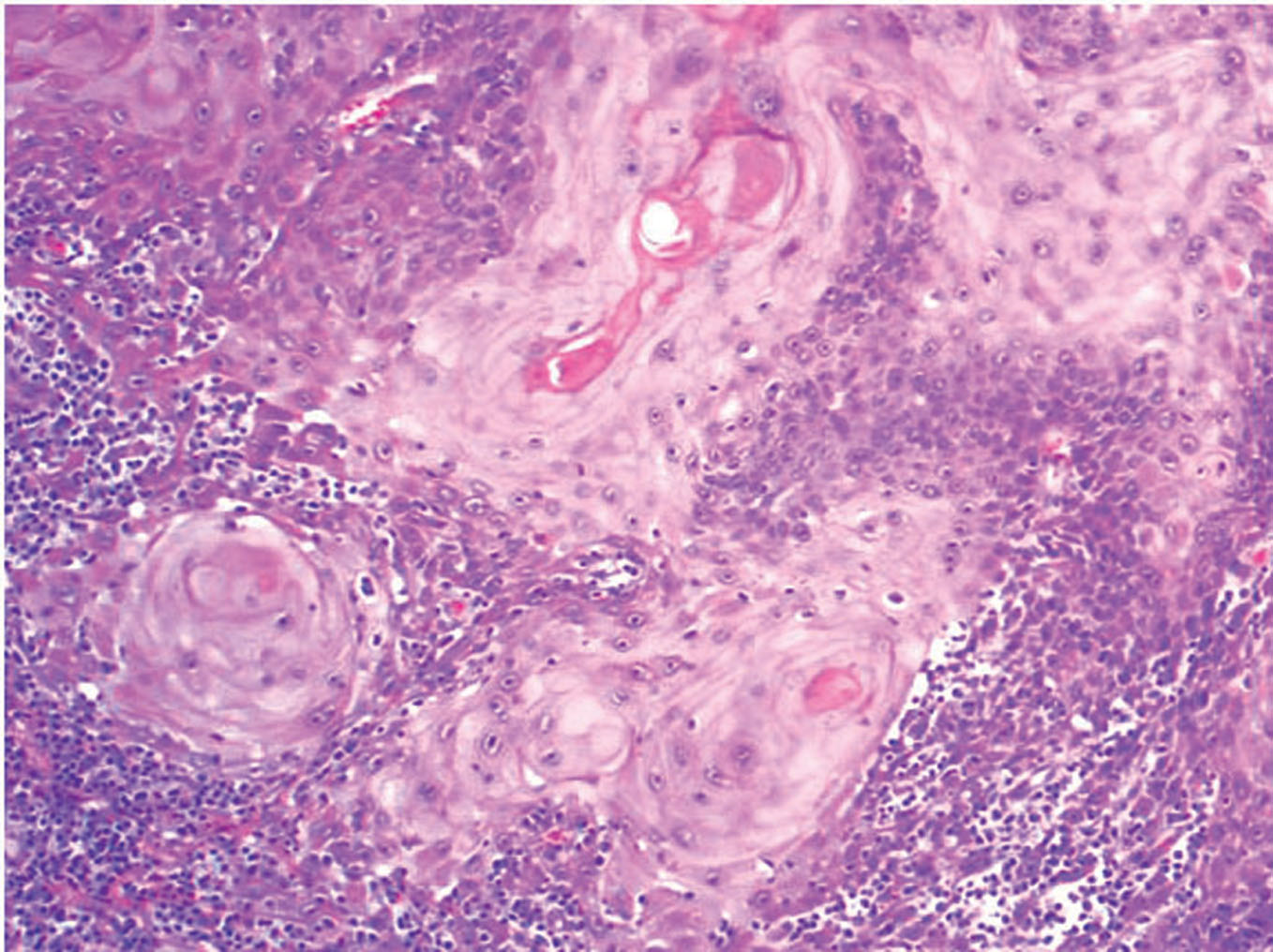 Figure 5. Invasive neoplastic cells with keratin pearl formation (H&E, 10x)
|
Discussion
Oculocutaneous albinism (OCA) is a group of four autosomal recessive disorders caused by either a complete lack or a reduction of melanin biosynthesis in the melanocytes resulting in hypopigmentation of the hair, skin and eyes. Reduction of melanin in the eyes results in reduced visual acuity caused by foveal hypoplasia and misrouting of the optic nerve fibres. The clinical spectrum of OCA varies, with OCA1A being the most severe type characterized by a complete lack of melanin production throughout life, while the milder forms OCA1B, OCA2, OCA3 and OCA4 show some pigment accumulation over time. The different types of OCA are caused by mutations in different genes but the clinical phenotype is not always distinguishable, making molecular diagnosis a useful tool and essential for genetic counseling [7]. Albinism can affect people of all ethnic backgrounds and has been extensively studied. Approximately one in 17,000 people have one of the types of albinism [8]. This suggests that about 1 in 70 people carry a gene for OCA [7]. Prevalence of the different forms of albinism varies considerably worldwide, partly explained by the different founder mutations in different genes and the fact that it can be difficult clinically to distinguish between the different subtypes of albinism among the large normal spectrum of pigmentation. OCA2 is the most prevalent form worldwide [9]. OCA1 is caused by mutations in the tyrosinase gene on chromosome 11q14.3 [10]. Mutations completely abolishing tyrosinase activity result in OCA1A, while mutations rendering some enzyme activity result in OCA1B allowing some accumulation of melanin pigment over time. Mutations in the OCA2 gene (formerly known as the P-gene) cause the OCA2 phenotype [11]. All types of OCA and ocular albinism (OA) have similar ocular findings, including various degrees of congenital nystagmus, hypopigmentation of iris leading to iris translucency, reduced pigmentation of the retinal pigment epithelium, foveal hypoplasia, reduced visual acuity usually in the range 20/60 to 20/400 and refractive errors, and sometimes a degree of color vision impairment [8,12]. Photophobia may be prominent. A characteristic finding is misrouting of the optic nerves, consisting in an excessive crossing of the fibres in the optic chiasma, which can result in strabismus and reduced stereoscopic vision [13]. Absence of misrouting excludes the diagnosis of albinism. The degree of skin and hair hypopigmentation varies with the type of albinism but is in general reduced [12]. In OCA1A the hair, eyelashes and eyebrows are white, and the skin is white and does not tan. Irises are light blue to almost pink, and fully translucent. Pigment does not develop and amelanotic nevi may be present. The symptoms do not vary with age or race. Visual acuity is 1/10 or less, and photophobia is intense. In OCA1B, the hair and skin may develop some pigment with time (after 1 to 3 years), and blue irises may change to green/brown. Visual acuity is 2/10. This phenotype was previously known as yellow albinism. In OCA2, the amount of cutaneous pigment may vary, and newborn nearly always have pigmented hair. Nevi and ephelids are common. Iris color varies and the pink eyes seen in OCA1A are usually absent. Visual acuity is usually better than in OCA1, and can reach 3/10 [7]. The diagnosis of OCA is based on clinical findings of hypopigmentation of the skin and hair, in addition to the characteristic ocular symptoms. However, due to the clinical overlap between the OCA subtypes, molecular diagnosis is necessary in order to establish the gene defect and thus the OCA subtype. Molecular genetic testing is based on mutational analysis of the genes, by standard screening methods such as denaturing high performance liquid chromatography (DHPLC) or single stranded conformational polymorphism (SSCP), followed by DNA sequencing [7]. The parents of an affected child are obligate carriers, the recurrence risk for another affected child is 25%, and healthy sibs are at 67% risk of being carriers. Offspring of an affected person are obligate carriers. Carriers are asymptomatic. Prenatal diagnosis can be done on DNA extracted from chorion villus sampling (CVS) at 10?12 weeks gestation or on DNA extracted from cultured amniocytes [7]. Prenatal diagnosis has been performed on skin biopsies from the fetus [14]. Skin cancers are generally commoner in the middle aged and elderly. In albinos however these cancers are known to present earlier [15-17]. Most important risk factor for the development of SCC is environmental exposure to ultraviolet light, as evidenced by increased incidence in sunnier climates, lower rates in dark skin & majority arising over sun-exposed skin. Melanin is a photo protective pigment, protecting the skin from the harmful effects of ultraviolet radiation. Its deficiency in people with albinism predisposes them to the harmful effects of ultraviolet radiation exposure, resulting in issues such as photophobia, decreased visual acuity, extreme sun sensitivity, and skin cancers [18]. High levels of exposure to ultraviolet radiation increase the risk of all three major forms of skin cancer and are responsible for the anatomical site distribution [19]. No use of protection for the skin increased the risk of skin cancer in these patients. Kromberg et al. reported that 23.4 % of albinos developed skin cancer out of 111 albinos studied in South Africa [5]. The head and the neck is the site most commonly affected and squamous cell carcinoma has been reported to be the commonest skin malignancy seen in albinos [5,20]. Sqamous cell carcinoma arising from actinic keratoses has been reported from India [21]. In the African albino, the risk of developing these malignancies in comparison to the general population has been reported to be as high as up to 1000 fold [22]. Surgery has been reported to be the mainstay of treatment of the majority of skin cancers in albinos [22,23]. Adequate surgical resection is most important to prevent local recurrence. Good results can be obtained with radical surgery and optimal surgical margins along with reconstructive procedure when needed [24]. From available reports, skin cancers in albinos are preventable [21,22]. There is therefore a need for early institution of skin protective measures in these patients which include protective clothing, sun-screening agents, indoor occupations, and early detection and treatment of skin cancers. For this to be effective: the public should receive education on early institution of preventive measures, register all albinos early in life and educate them to prevent the damaging effect of the sun (protective clothing, sun-screening agents and indoor occupations). Regular examination of all albinos for early detection and treatment of the various malignant lesions to which they are prone deserves to be included in the current anti-cancer campaign, to which the medical world is committed [21]. Dermatologists should maintain a high index of suspicion in this vulnerable population.
Conclusion
Albinism and solar radiation are risk factors for skin cancer. Early implementation of public education strategies on skin cancer prevention is an important long term goal which should improve the outcome in these albinos. Providing free annual skin check up would improve early detection and treatment, hence reducing the morbidity and mortality of skin cancers in these patients.
REFERENCES
1. Witkop CJ: Albinism. Clin Dermatol. 1989;7:80?91. 2. Summers G: Albinism: classification, clinical characteristics and recent findings. Optom Vis Sci. 2009;86:659?62. 3. Opara KO, Jiburum BC: Skin cancers in albinos in a teaching Hospital in eastern Nigeria- presentation and challenges of care. World Journal of Surgical Oncology. 2010;8:73. 4. Yakubu A, Mabogunje OA: Skin cancer in African albinos. Acta Oncol. 1993;32:621?2. 5. Kromberg JG, Castle D, Zwane EM, Jenkins T: Albinism and skin cancer in Southern Africa. Clin Genet. 1989;36:43?52. 6. Lookingbill DP, Lookingbill GL, Leppard B: Actinic damage and skin cancer in albinos in northern Tanzania: findings in 164 patients enrolled in an outreach skin care program. J Am Acad Dermatol. 1995;32:653?8. 7. Karen Gr?nskov, Jakob Ek, Karen Brondum-Nielsen: Oculocutaneous albinism. Orphanet J Rare Dis. 2007;2:43. 8. Witkop CJ: Albinism: hematologic-storage disease, susceptibility to skin cancer, and optic neuronal defects shared in all types of oculocutaneous and ocular albinism. Ala J Med Sci. 1979;16:327-30. 9. Lee ST, Nicholls RD, Schnur RE, Guida LC, Lu-Kuo J, Spinner NB, et al: Diverse mutations of the P gene among African-Americans with type II (tyrosinase-positive) oculocutaneous albinism (OCA2). Hum Mol Genet. 1994;3:2047-51. 10. Tomita Y, Takeda A, Okinaga S, Tagami H, Shibahara S: Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem Biophys Res Commun. 1989;164:990-6. 11. Rinchik EM, Bultman SJ, Horsthemke B, Lee ST, Strunk KM, Spritz RA, et al: A gene for the mouse pinkeyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993;361:72-6. 12. King RA, Summers CG: Albinism. Dermatol Clin. 1988;6:217-28. 13. Creel D, O?Donnell FE Jr., Witkop CJ Jr.: Visual system anomalies in human ocular albinos. Science. 1978;201:931-3. 14. Rosenmann E, Rosenmann A, Ne?eman Z, Lewin A, Bejarano-Achache I, Blumenfeld A: Prenatal diagnosis of oculocutaneous albinism type I: review and personal experience. Pediatr Dev Pathol. 1999;2:404-14. 15. Hong ES, Zeeb H, Repacholi MH: Albinism in Africa as a public Health issue. BMC Publ Health. 2006;6:212. 16. Yakubu A, Mabogunje OA: Skin cancer in African albinos. Acta Oncol. 1993;32:621?2. 17. Alexander GA, Henschke UK: Advanced skin cancers in Tanzanian Albinos: preliminary observations. J Natl Med Assoc. 1981;73:1047?54. 18. Lookingbill DP, Lookingbill GL, Leppard B: Actinic damage and skin cancer in albinos in northern Tanzania: findings in 164 patients enrolled in an outreach skin care program. J Am Acad Dermatol. 1995;32:653?8. 19. Glanz K, Saraiya M: Using evidence-based community and behavioural interaction to prevent skin cancer. Opportunities and challenges for Public Health Practice. Prev Chronic Dis. 2005;2:1?5. 20. Yakubu A, Mabogunje OA: Skin cancer in African albinos. Acta Oncol. 1993;32:621?622. 21. Ramalingam VS, Sinnakirouchenan R, Thappa DM: Malignant transformation of actinic keratoses to squamous cell carcinoma in an albino. Indian J Dermatol. 2009;54:46?8. 22. Hong ES, Zeeb H, Repacholi MH: Albinism in Africa as a public Health issue. BMC Publ Health. 2006;6:212. 23. Berger E, Hunt R, Tzu J, Patel R, Sanchez M: Squamous-cell carcinoma in situ in a patient with oculocutaneous albinism. Dermatol Online J. 2011;17:22. 24. Mabula JB, Chalya PL, Mchembe MD, Jaka H, Giiti G, Rambau P, et al: Skin cancers among Albinos at a University teaching hospital in Northwestern Tanzania: a retrospective review of 64 cases. BMC Dermatol. 2012;12:5.
Comments are closed.